4
III. NOTICE - READ BEFORE USE
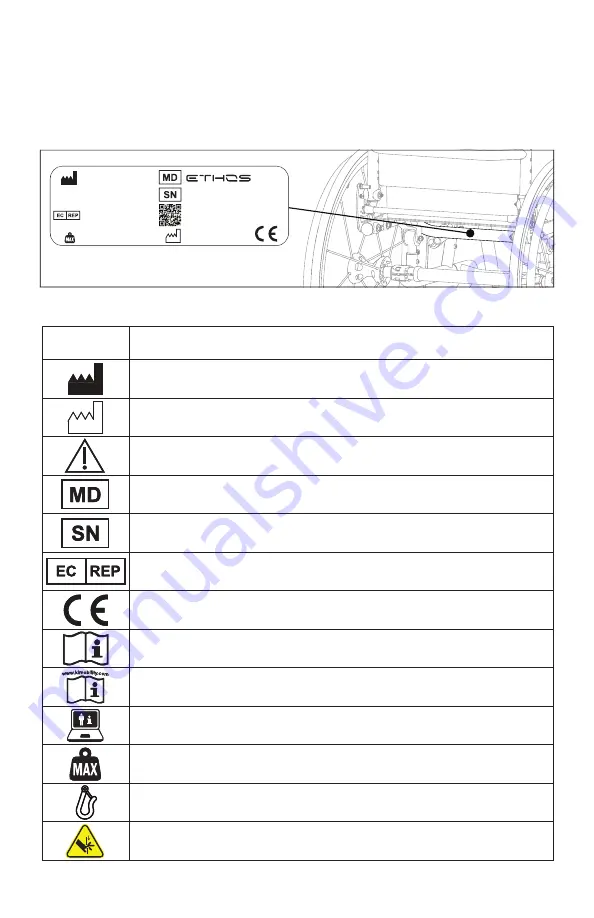
D. Serial Number Identification
The serial number label provides important information about your chair and the serial number is
used as the unique identifier for your specific chair. See image below on where to find the serial
number on your chair.
E. Symbol Glossary
ETXXXXXXX
Ki Mobility
5201 Woodward Drive
Stevens Point, WI 54481
(715) 254-0991
www.kimobility.com
275 LBS (125 KG)
(01)00850013379095
(11)YY/MM/DD
(21)ETXXXXXXX
YEAR/MM/DD
Ki Mobility
5201 Woodward Drive
Stevens Point, WI 54481
SYMBOL
DEFINITION
Indicates the medical device manufacturer.
Indicates the need for the user to consult an instruction for safety reasons such as cautions and
warnings. If presented on the medical device or packaging, it indicates the need for the user to
consult the instructions for safety reasons such as cautions and warnings.
Indicates the item is a medical device.
Indicates the manufacturer’s serial number so that a specific medical device can
be identified.
Indicates the authorized representative in the European Community.
Indicates the manufacturer’s declaration that the product meets the require-
ments of the applicable EC directives.
Indicates the need for the user to consult the instructions for use.
Indicates the need for the user to consult the listed website for instructions for
use in an electronic format.
Indicates a website where a user may obtain additional information about the
medical product.
Indicates a specified maximum weight limit (lbs/kg).
Indicates a transit securement point.
Indicates a potential pinch point.
Date of manufacture (YEAR/MM/DD).
SYMBOL
DEFINITION
Indicates the medical device manufacturer.
Indicates the need for the user to consult an instruction for safety reasons such as cautions and
warnings. If presented on the medical device or packaging, it indicates the need for the user to
consult the instructions for safety reasons such as cautions and warnings.
Indicates the item is a medical device.
Indicates the manufacturer’s serial number so that a specific medical device can
be identified.
Indicates the authorized representative in the European Community.
Indicates the manufacturer’s declaration that the product meets the require-
ments of the applicable EC directives.
Indicates the need for the user to consult the instructions for use.
Indicates the need for the user to consult the listed website for instructions for
use in an electronic format.
Indicates a website where a user may obtain additional information about the
medical product.
Indicates a specified maximum weight limit (lbs/kg).
Indicates a transit securement point.
Indicates a potential pinch point.
Date of manufacture (YEAR/MM/DD).




































