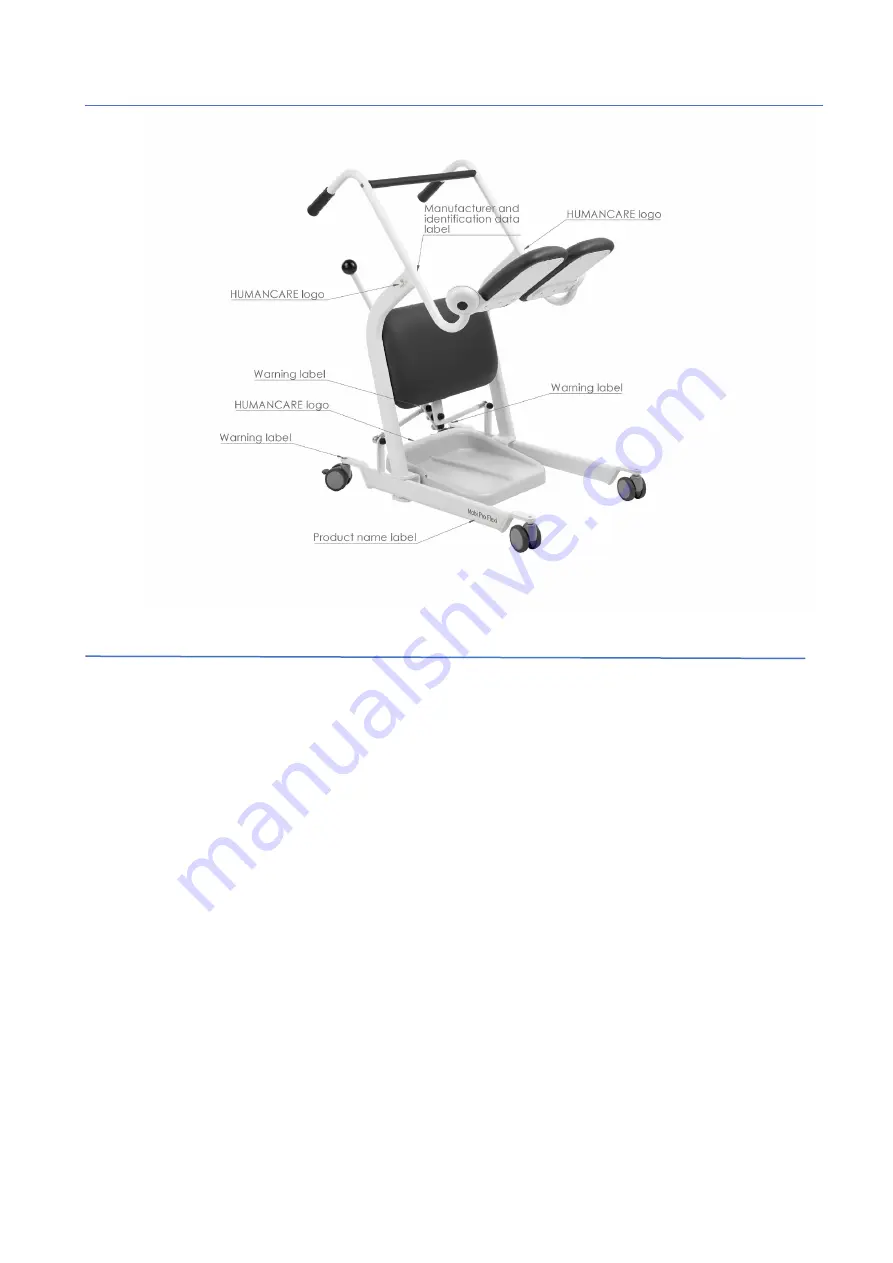
20
Label placement
Approvals and List of Standards
Product complies with the European Regulation (EU) 2017/745 of the European Parliament
and of the Council of 5 April 2017 on medical devices:
- Classified as Class I medical devices as set out in Annex VIII of Regulation (EU) 2017/745
- Complies with the provisions of Regulation (EU) 2017/745 “Medical Devices” and meets
the essential requirements set out in Annex I of Regulation (EU) 2017/745
- The conformity for the purposes of the CE marking and conformity assessment according
to the procedure from Annex IV of Regulation (EU) 2017/745
- The product also complies to the requirements of the following standards:
•
EN 12182:2012,
•
EN ISO 15223-1:2017-02 (eq. EN ISO 15223-1:2016),
•
EN ISO 14971:2019
















































