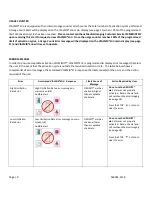
DEFINITY® is ready for use if VIALMIX®
RFID
shakes the vial uninterrupted for the full activation cycle with no error
messages displayed. The following screen will be displayed indicating a successful activation:
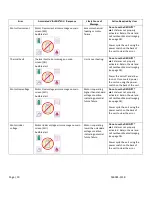
If the DEFINITY® vial does not shake for the full 45 second duration or at the acceptable rate, activation is not considered
successful and the vial must not be used. An error message will be displayed and an audible alarm will sound to alert
the user. Refer to page 9 for a list of error messages and the conditions they indicate.
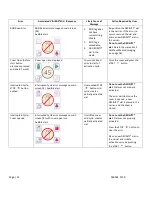
If an interruption in power (power failure) occurs during an activation cycle, the activation is not considered
successful and the DEFINITY® vial must not be used. Once the power is restored, power cycle the unit using the
power switch located on the back of the unit. A new DEFINITY® vial is required for activation.
Removing the DEFINITY® Vial
After successful activation of DEFINITY®, the vial can be removed for use. To remove the vial, open the cover and follow
these steps:
1.
Press the lever down to unlatch the vial carrier then press the lever to the left to fully open the carrier.
2.
Remove the DEFINITY® vial from the carrier.
3.
Release the lever and the spring action will close the carrier.
4.
Close the cover.
Powering Off VIALMIX®
RFID
VIALMIX®
RFID
should be powered off at the end of the day. This is done by moving the switch on the back of the unit to
the "O" position.
Page | 8
516041-1118
Summary of Contents for VIALMIX RFID
Page 19: ...Page 19 516041 1118...