Operating Manual MELAdoc label printer
6
Storage
Loss of sterility
is dependent less on the length of the storage time as from external
influences during storage, as well as transport and handling. When opening the
packaging, dust and microorganisms deposited on the packaging during the storage
time can fall on the instruments, thus contaminating them. An ideal storage time can
thus not be generally specified.
Specification
of a suitable storage time is to be taken from the hygiene plan.
Responsibility for the storage conditions and length rests with the practice operator.
Damage to the sterilization packaging
usually follows isolated events and is not a
factor of time. Primary and secondary packaging may only be opened immediately
prior to use. Remove all dust on the packaging before doing so.
Primary packaging
is the sealed packaging system surrounding the medical product
and holding it sealed from all germs (DIN EN 868-1:1997-05).
Secondary packaging
is the packaging containing one or more medical products,
each of which enclosed in its own primary packaging (DIN EN 868-1:1997-05).
Responsibility
for maintaining the specified storage requirements and times rests
with the facility operator.
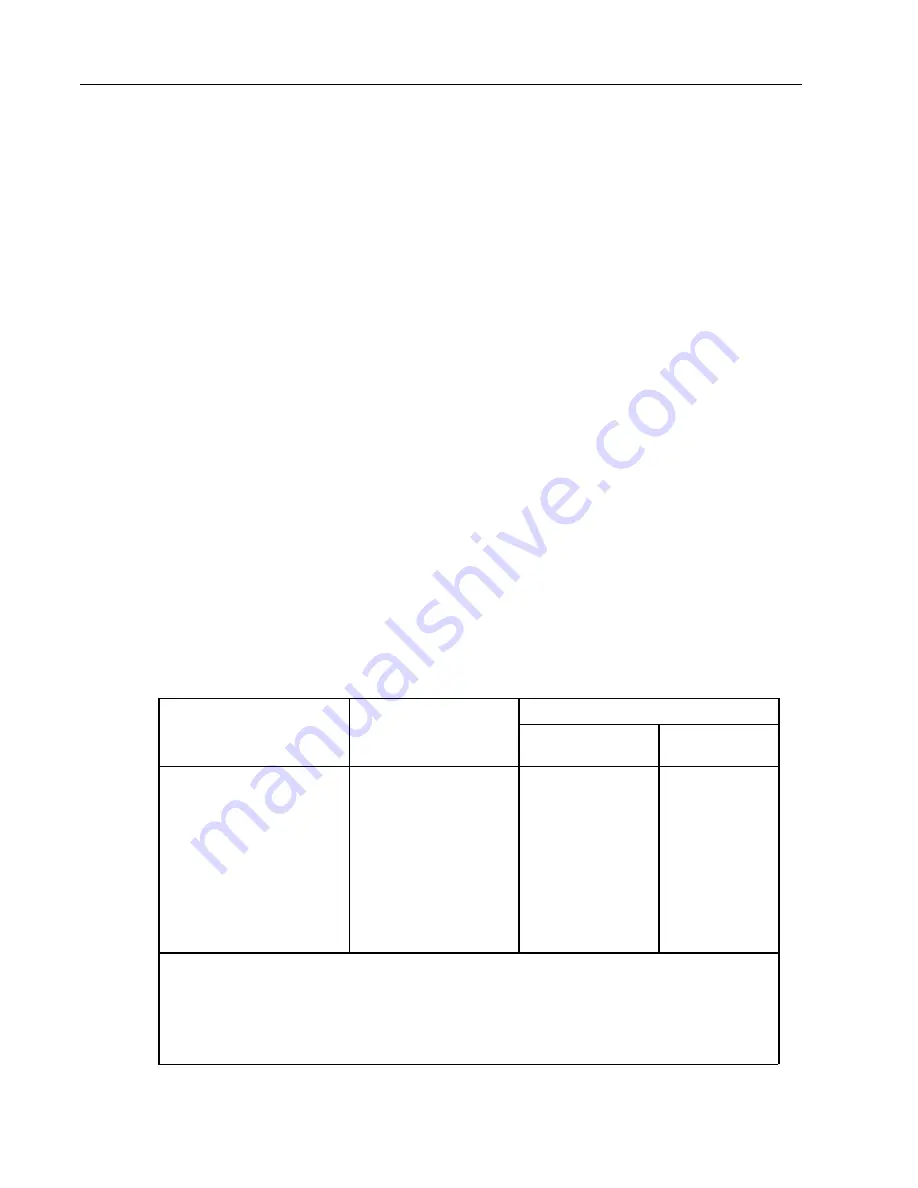
Storage length according to DIN 58953-8 from October 2003:
Storage period
Sterilized material
packaging
Packaging type
Unprotected
storage
1
Protected
storage
Paper bag in
accordance with DIN
EN 868-4 and heatable,
self-sealing transparent
bag and tubing of paper
and plastic composite
film in accordance with
DIN EN 868-5, or other
equivalent packaging.
Sterilized material in
primary or secondary
packaging
Serves provision
for immediate
use.
2
To be avoided
as method of
storage
6 months,
although no
longer than
expiry date
3
1)
On shelves in rooms which do not correspond with room class 1 as defined by DIN 1946-4
(Ventilation air conditioning) 1999-03, table 2
2)
Immediate use means application / use of the product within a maximum of 2 days / 48
hours.
3)
Experience has shown that exceeding the storage period when using this type of package
is not to be recommended for both practical and economical reasons.



































