2B-2 - ELECTRICAL
90-852396 MAY 1996
Battery
Precautions
WARNING
If battery acid comes in contact with skin or eyes,
wash skin immediately with a mild soap. Flush
eyes with water immediately and see a doctor.
When charging batteries, an explosive gas mixture
forms in each cell. Part of this gas escapes through
holes in vent plugs and may form an explosive atmo-
sphere around battery if ventilation is poor. This ex-
plosive gas may remain in or around battery for sev-
eral hours after it has been charged. Sparks or
flames can ignite this gas and cause an internal ex-
plosion which may shatter the battery.
The following precautions should be observed to pre-
vent an explosion.
1. DO NOT smoke near batteries being charged or
which have been charged very recently.
2. DO NOT break live circuits at terminals of batter-
ies because a spark usually occurs at the point
where a live circuit is broken. Always be careful
when connecting or disconnecting cable clamps
on chargers. Poor connections are a common
cause of electrical arcs which cause explosions.
3. DO NOT reverse polarity of battery terminal to
cable connections.
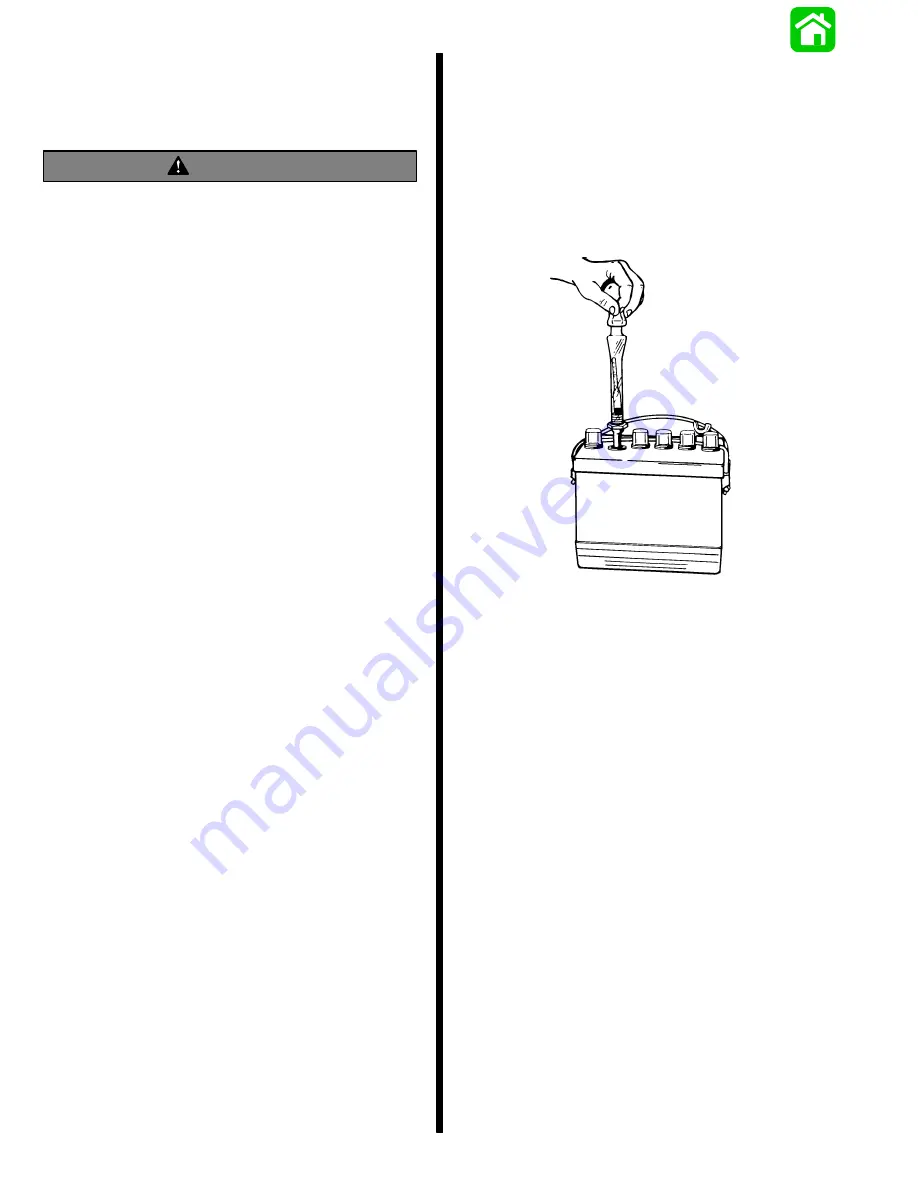
Specific Gravity Readings
Use a hydrometer to measure specific gravity of elec-
trolyte in each cell.
Hydrometer measures percentage of sulfuric acid in
battery electrolyte in terms of specific gravity. As a
battery drops from a charged to a discharged condi-
tion, acid leaves the solution and enters the plates,
causing a decrease in specific gravity of electrolyte.
An indication of concentration of electrolyte is ob-
tained with a hydrometer.
When using a hydrometer, observe the following
points:
1. Hydrometer must be clean (inside and out) to in-
sure an accurate reading.
2. Never take hydrometer readings immediately af-
ter water has been added. Water must be thor-
oughly mixed with electrolyte by charging for at
least 15 minutes at a rate high enough to cause
vigorous gassing.
3. If hydrometer has built-in thermometer, draw liq-
uid in several times to ensure correct tempera-
ture before taking reading.
4. Hold hydrometer vertically and draw in just
enough liquid from battery cell so that float is free-
floating. Hold hydrometer at eye level so that float
is vertical and free of outer tube, then take read-
ing at surface of liquid. Disregard curvature
where liquid rises against float stem due to capil-
larity.
22532
5. Avoid dropping electrolyte on boat or clothing, as
it is extremely corrosive. Wash off immediately
with baking soda solution.
Specific gravity of electrolyte varies not only with per-
centage of acid in liquid but also with temperature. As
temperature increases, electrolyte expands, so that
specific gravity is reduced. As temperature drops,
electrolyte contracts, so that specific gravity in-
creases. Unless these variations in specific gravity
are taken into account, specific gravity obtained by
hydrometer may not give a true indication of acid in
electrolyte.
A fully charged battery will have a specific gravity
reading of approximately 1.270 at an electrolyte tem-
perature of 80
°
F (27
°
C). If electrolyte temperature
is above or below 80
°
F, additions or subtractions
must be made in order to obtain a hydrometer read-
ing corrected to 80
°
F standard. For every 10
°
F (3.3
°
C) above 80
°
F, add 4 specific gravity points (.004) to
hydrometer reading. Example: A hydrometer reading
of 1.260 at 110
°
F (43
°
C) would be 1.272 corrected
to 80
°
F, indicating a fully charged battery.
For every 10
°
below 80
°
F, subtract 4 points (.004)
from the reading. Example: A hydrometer reading of
1.272 at 0
°
F (-18
°
C) would be 1.240 corrected to 80
°
F, indicating a partially charged battery.





































