QXMédical, LLC
Document Part Number: 3064-001, Rev. 06 (10/2019)
Page 3 of 76
Instructions For Use
1. DEVICE DESCRIPTION
The Stent Graft Balloon Catheter is designed for temporary
occlusion of large vessels and to assist in the expansion of stent
grafts used for the treatment of aortic aneurysms. Ballooning of a
stent graft may improve modeling of the graft material and fixation
of the stent graft to the vessel wall. Sub-optimal expansion of stent
grafts may also be improved by inflating the balloon at the site
of the stent. The Stent Graft Balloon Catheter has been tested to
temporarily occlude vessels up to 41 mm in diameter.
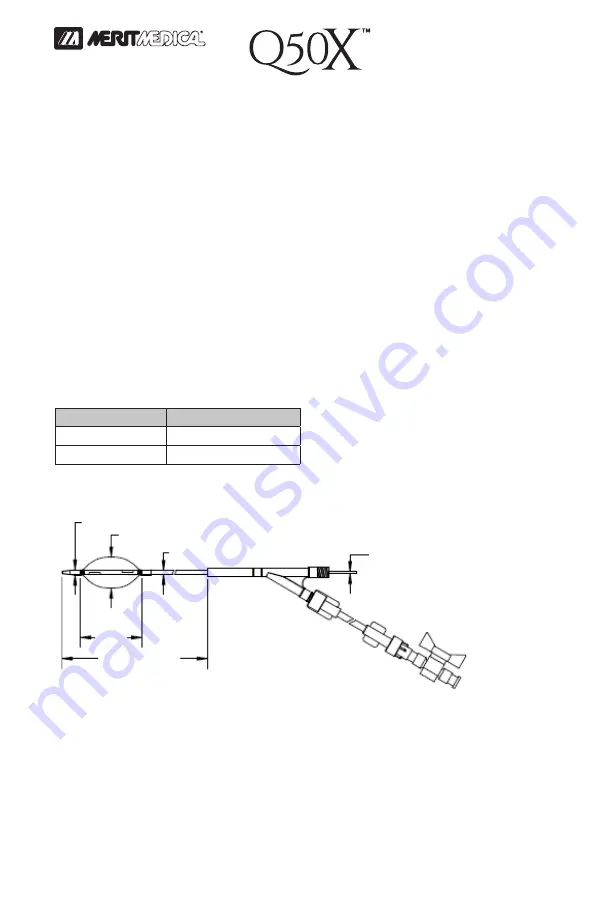
The Stent Graft Balloon Catheter is an over-the-wire (OTW) tri-
lumen catheter with a compliant polyurethane balloon having
a maximum diameter of 50 mm at 60 cc. Two lumens inflate and
deflate the balloon while one lumen is reserved for guidewire
passage. The catheter is offered in two (2) usable lengths, 100 cm
and 65 cm.
This device is designed to accommodate a 0.038” diameter (or
smaller) guidewire. Two (2) radiopaque marker bands are placed
within the balloon at each end [40 mm apart] to facilitate balloon
placement prior to inflation. The catheter can accommodate a
0.038” diameter (or smaller) guidewire and is compatible with 10 Fr
(or larger) introducer sheaths. The catheter has an extension tube
with a stopcock in order to facilitate handling and fluid control. The
device models are listed below:
Catalogue Number
Catheter Effective Length
Q50-65-X
65 cm
Q50-100-X
100 cm
There are risks involved with any medical procedure. Both physician
and patient should fully understand those risks associated with
surgery, and additional new risks associated specifically with the
use of this endoluminal device.
CAUTION: Only physicians trained in vascular surgery,
interventional radiology or cardiology, and who have
completed training or have experience with stent grafts,
balloon catheters and associated devices should consider
using this device.
CAUTION: Read the entire Instructions for Use manual prior to
using the device.
8 Fr (2.70 mm)
10 Fr (3.33 mm) Minimum Sheath Diameter
1.97" (50 mm) Maximum Balloon Diameter
0.038" (0.97 mm)
Maximum Guidewire
Diameter
40 mm
65 cm OR 100 cm
Q50
®
X
STENT GRAFT
BALLOON CATHETER
2. INDICATIONS FOR USE
The Stent Graft Balloon Catheter is intended for temporary
occlusion of large vessels or to expand vascular prostheses.
3. CONTRAINDICATIONS
The Stent Graft Balloon Catheter is contraindicated in patients
who:
• Are contraindicated to contrast media or anticoagulants
• Have an arterial entry site that cannot accommodate a 10 Fr
(min) introducer sheath
• Are minors <18 years old
• Are pregnant
4. WARNINGS
• The Stent Graft Balloon Catheter is supplied STERILE and for
single use only. Do not reprocess or re-sterilize. Reprocessing
and re-sterilizing could increase the risk of patient infection
and of compromised device performance.
• The catheter should only be manipulated and inflated/deflated
while observing under fluoroscopy.
• If resistance is encountered at any time during the insertion
procedure, do not force passage or torque the catheter.
Resistance may cause damage to device, vessel or stent graft.
Carefully withdraw the catheter.
• Do not torque or twist the catheter during insertion or
withdrawal.
• The catheter should only be advanced or withdrawn over a
guidewire.
• Adhere to balloon inflation parameters outlined in the
Balloon Compliance Chart (Table 1)
. Do not exceed a balloon
diameter of 50 mm and do not exceed 60 cc inflation volume at
50 mm balloon diameter. Rupture of balloon may occur. Over-
inflation may result in damage to vessel wall and/or vessel
rupture, or damage to the stent graft.
• Balloon rupture may occur under certain anatomical, procedural,
and/or clinical circumstances. It is therefore recommended to
have back-up Stent Graft Balloon Catheters on hand.
• Ensure that the balloon is fully deflated before moving the
Stent Graft Balloon Catheter.
• When expanding a vascular prosthesis, there is an increased
risk of vessel injury and/or rupture, and possible patient death,
if the balloon inflation is not completely within the covered
(graft fabric) portion of the prostheses.
• Studies indicate that the danger of micro-embolization
increases with increased manipulation and/or duration of the
procedure.
• Over inflation of the balloon can cause graft tears and/or vessel
rupture. Care should be taken when inflating the balloon in




































