COMPRESSION DEVICE
I N S T R U C T I O N S F O R U S E
PRODUCT DESCRIPTION
Merit Medical’s SAFEGUARD Radial™ compression device is a sterile, single
use disposable device. It has a clear medical grade polyurethane window and
bulb (balloon) that facilitates visualization of the puncture site, a clear medical
grade PVC flexible tube, and a pressure sensitive, self-adhesive peel backing.
A valve on the end of the fill tube enables a standard luer or proprietary
syringe to be connected to inflate and deflate the bulb with air to provide
compression of the transradial puncture site. Product not made with natural
rubber latex.
INDICATIONS FOR USE
SAFEGUARD Radial is a compression device to assist hemostasis of the radial
artery after a transradial procedure.
CONTRAINDICATIONS
The adhesive portion of SAFEGUARD Radial should not be used over excori-
ated skin.
WARNINGS
• Prior to inflation of bulb, confirm that you are ONLY injecting air into
SAFEGUARD Radial compression device and NOT to side port of sheath or
other device.
• Over-inflation of bulb (above 7 mL of air) could cause bulb to excessively
expand and cause pain, numbness, or radial artery occlusion.
• Under-inflation of bulb could compromise ability of device to assist hemo-
stasis of radial artery.
• Maintain sterile field during application.
CAUTIONS
• Read instructions prior to use.
• RX Only: Caution: Federal (USA) law restricts this device to sale by or on the
order of a physician.
• This device is intended for single use only. Do not reuse or resterilize.
• Do not use if package is damaged
• This device should be used by clinicians with adequate training in the use
of the device.
REUSE PRECAUTION STATEMENT
For single patient use only. Do not reuse, reprocess or resterilize. Reuse,
reprocessing or resterilization may compromise the structural integrity of the
device and/or lead to device failure which, in turn, may result in patient injury,
illness or death. Reuse, reprocessing or resterilization may also create a risk of
contamination of the device and/or cause patient infection or cross-infection,
including, but not limited to, the transmission of infectious disease(s) from
one patient to another. Contamination of the device may lead to injury, illness
or death of the patient.
POTENTIAL COMPLICATIONS
Possible complications that may result from use of this device include, but are
not limited to: hematoma; bleeding; pain or numbness; radial artery occlusion.
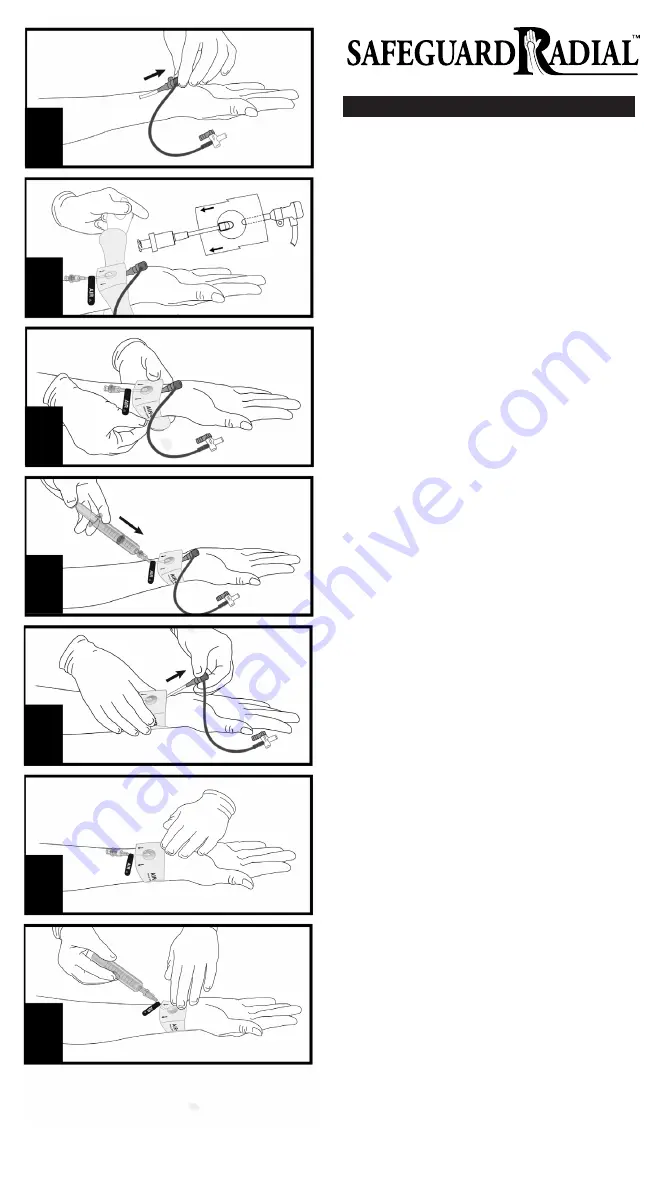
INSTRUCTIONS FOR USE
1. Ensure site is clean and dry.
2. After procedure, aspirate sheath, then withdraw sheath one inch (2-3 cm).
(Figure 1)
3. Peel back adhesive backing of SAFEGUARD Radial compression device
approximately halfway (both sides), then apply center of bulb over arteri-
otomy site. (Figure 2)
Note
: The arrows on SAFEGUARD Radial should be pointing up the arm of
the patient.
4. Remove remainder of adhesive backing (one side at a time) and complete-
ly secure around wrist. (Figure 3)
5. Attach and completely engage a standard luer or proprietary syringe to
the tubing line labeled “AIR”.
WARNING
: Prior to inflation of bulb, confirm that you are ONLY injecting air
into SAFEGUARD Radial compression device and NOT to side port of sheath
or other device.
6. Inflate the bulb (balloon) with a maximum volume of 7 mL of air.
(Figure 4) Remove syringe.
WARNING
: Over-inflation of bulb above 7 mL of air could cause bulb to exces-
sively expand and cause pain, numbness, or radial artery occlusion.
WARNING
: Under-inflation of bulb could compromise ability of device to
assist hemostasis of radial artery.
7. Remove sheath, then confirm there is no bleeding from puncture site by
viewing site through bulb window. If bleeding is observed at any time,
inject more air (not exceeding the maximum inflate volume) until bleeding
stops. (Figure 5)
8. Per hospital protocol, ensure adequate distal perfusion is maintained. If
necessary, adjust air volume in bulb. (Figure 6)
Note
: Air volume and compression time may differ according to patient’s
condition, anticoagulant dosage, and size of puncture site.
9. Before removing device, confirm that bleeding has stopped. Attach
syringe to tubing (holding plunger in place). Slowly deflate bulb. (Figure 7)
Note
: Be sure not to create a vacuum by pulling back on the plunger too fast.
10. Carefully remove SAFEGUARD Radial compression device and apply sterile
dressing per hospital protocol.
1
2
3
4
5
6
7
1
2
3
4
5
6
7































