CorrTran MV Corrosion Monitor
51
9 MEASURING PRINCIPLE
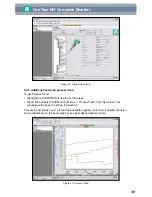
Figure 44. CorrTran MV Corrosion Cell
General corrosion
Linear Polarization Resistance (LPR) is based on the fact that in a corroding electrode the
relationship between i
corr
and the polarization resistance is give by the following equation:
i
corr
= B
R
p
, where
R
p
= ∆E
∆I
with
∆E
being the applied voltage and
∆I
the resulting current.
Harmonic Distortion Analysis (HDA) allows CorrTran MV to determine i
corr
without using the
Tafel slopes (ba, bc). This is typically done by applying a low frequency sinusoidal voltage
and determining the distance of the resulting current.
CorrTran MV accurately measures the general corrosion rate by implementing Harmonic
Distortion Analysis (HDA) to improve the performance of Linear Polarization Resistance
(LPR). A process-specific Stern-Geary voltage (Bharm) is calculated with every
measurement cycle through HDA. This value is then implemented in the LPR corrosion
rate calculation resulting in a highly accurate self adjusting, process specific corrosion rate
calculation. CorrTran MV can measure the general corrosion rate from 0 … 1000 mpy (0 …
25 mmpy).
Localized Corrosion (Pitting)
Electrochemical Noise (ECN) is the method of monitoring spontaneous fluctuations
generated at the interface of the corroding metal and process solution. As localized
corrosion occurs, these fluctuations increase.
The CorrTran MV monitors for these fluctuations on the electrode surfaces for 17 minutes.
It then performs a statistical analysis resulting in a unitless pitting factor value between
0 and 1. A pitting factor of nearly 0 represents no localized corrosion activity and a pitting
factor of 1 represents high localized corrosion activity. Independent studies have revealed
that a sustained pitting factor of greater than 0.3 is cause for concern and you should
investigate the source of the elevated Localized Corrosion rate. For more information on
the interpretation of localized corrosion please review our white paper: “Detecting and
Interpreting Localized Corrosion Using CorrTran MV.”
Fe
Anodic
site
Cathodic
site
Fe
2+
2H
+
H
2
I
corr
2e
-