12
SySTEM
3.3.2 ANALySIS OF THE DRyING PROCESS
Drying occurs when a sample loses water. When water loss stops, under a specified set of
conditions, the sample is said to be dry. Heating is often assumed to be necessary for drying,
but high temperatures are only peripherally related to drying.
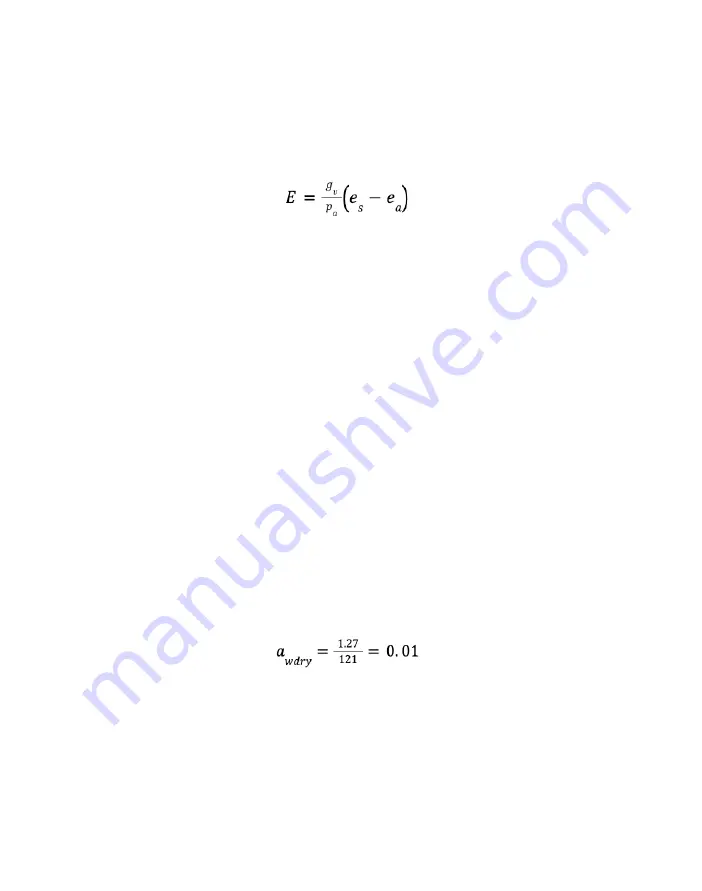
The integrated form of the Fick equation (
) gives the rate of water loss from a sample:
Equation 1
where:
E
=
the evaporation rate (
g
×
m
-2
s
-1
)
When the sample is dry,
E
becomes 0
NOTE: The sample and air-vapor pressures are equal.
g
v
=
the vapor conductance of the sample surface and the air surrounding the sample
(
g
×
m
-2
s
-1
)
p
a
=
the atmospheric pressure (kPa)
e
a
and
e
s
=
the vapor pressures of the air (kPa) and of water at the sample surface, respectively.
d
=
the characteristic dimension
In a well-ventilated oven, the vapor pressure of the air in the oven (
e
a
) equals the vapor
pressure of the air in the laboratory where the oven resides. The vapor pressure of air is
the product of the air humidity (expressed as a fraction) and the saturation vapor pressure
at air temperature. In a typical laboratory, it is assumed that the relative humidity is 0.4 and
the air temperature is 25 °C, the vapor pressure of the oven air would be 1.27 kPa (0.4
×
3.17 kPa = 1.27 kPa, where 3.17 is the saturation vapor pressure (kPa) of air at 25 °C).
The vapor pressure of the sample (
e
s
) is the product of its water activity and the saturation
vapor pressure at sample temperature. A typical standard drying oven temperature is
105 °C. The saturation vapor pressure of water at that temperature is 121 kPa. Knowing
the saturation vapor pressure of water and the vapor pressure of the oven air can be used
to calculate the water activity of a dry sample.
shows the calculation for the
specified conditions:
Equation 2
The sample dry water activity can be computed for any vapor pressure that might exist in the
oven as shown in