B - 6
a
The ISM (industrial, scientific, and medical) bands between 150 kHz and 80 MHz are 6.765
MHz to 6.795 MHz; 13.553 MHz to 13.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to
40.70 MHz. The amateur radio bands between 0,15 MHz and 80 MHz are 1,8 MHz to 2,0 MHz,
3,5 MHz to 4,0 MHz, 5,3 MHz to 5,4 MHz, 7 MHz to 7,3 MHz, 10,1 MHz to 10,15 MHz, 14 MHz
to 14,2 MHz, 18,07 MHz to 18,17 MHz, 21,0 MHz to 21,4 MHz, 24,89 MHz to 24,99 MHz, 28,0
MHz to 29,7 MHz and 50,0 MHz to 54,0 MHz.
b
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV
broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the device is used exceeds
the applicable RF compliance level above, the device should be observed to verify normal
operation. If abnormal performance is observed, additional measures may be necessary,
such as re-orienting or relocating the device.
c
Over the frequency ranges 150 kHz to 80 MHz, field strengths should be less than 3V/m.
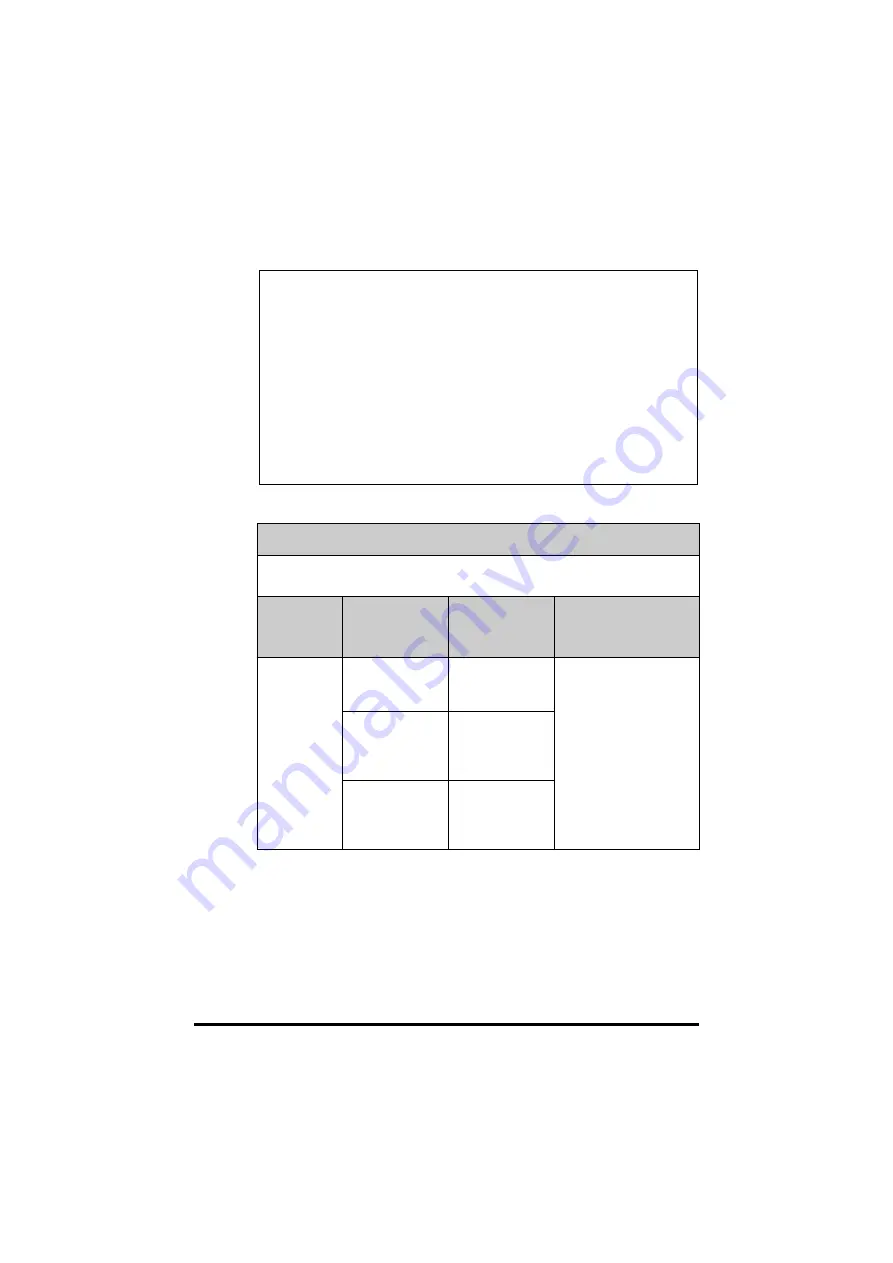
GUIDANCE AND MINDRAY DECLARATION - ELECTROMAGNETIC IMMUNITY
The device is intended for use in the electromagnetic environment specified below. The
customer or the user of system should assure that it is used in such an environment.
IMMUNITY
TEST
IEC 60601 TEST
LEVEL
COMPLIANCE
LEVEL
ELECTROMAGNETIC
ENVIROMENT -
GUIDANCE
Proximity
magnetic
fields
IEC 61000-4-39
8 A/m
30 kHz
CW
8 A/m
30 kHz
CW
/
65 A/m
134,4 kHz
Pulse modulation
2,1 kHz
65 A/m
134,4 kHz
Pulse modulation
2,1 kHz
7,5 A/m
13,56 MHz
Pulse modulation
50 kHz
7,5 A/m
13,56 MHz
Pulse modulation
50 kHz
Summary of Contents for BeneFusion uVP
Page 1: ...BeneFusion uVP BeneFusion uVP ex Infusion Pump Instruction for Use ...
Page 2: ......
Page 12: ...1 4 This page intentionally left blank ...
Page 18: ...3 4 This page intentionally left blank ...
Page 26: ...6 2 This page intentionally left blank ...
Page 30: ...8 2 This page intentionally left blank ...
Page 32: ...9 2 This page intentionally left blank ...
Page 36: ...10 4 This page intentionally left blank ...
Page 38: ...11 2 This page intentionally left blank ...
Page 40: ...12 2 This page intentionally left blank ...
Page 60: ...B 10 This page intentionally left blank ...
Page 61: ......
Page 62: ...KF H 046 024481 00 4 0 ...







































