Page 3 of 6
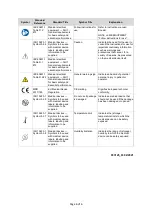
Warnings and Precautions:
WARNING
System components immersed or in contact with liquids may cause electrical shock.
•
Do not immerse, drip, or spray liquids onto the device.
CAUTION
Device dropped or damaged in transit/use could lead to loss of function or delayed diagnosis.
•
Inspect the device prior to each use and do not use if damaged.
Device when used by untrained user could lead to delayed diagnosis.
•
This device is intended to be used by qualified healthcare professionals.
Unauthorized modification, servicing or use of non Natus approved supplies or components
could lead to loss of device function or performance.
•
Do not modify device or use unauthorized supplies or components.
Environmental Specifications:
Operating Conditions:
•
Temperature: +15.6°C to +32.2°C (+60°F to +90°F)
•
Relative Humidity: 20% to 80% (non-condensing)
•
Altitude: 0 to 10,000 ft (0 to 3 km)
Storage Conditions:
•
Temperature: -17.7°C to +55°C (0°F to +132°F)
•
Relative Humidity: 10% to 90% (non-condensing)
•
Altitude: 0 to 35,000 ft (0 to 10.668 km)
Compliance Standards:
•
ISO 10993-1: 2018 Biological evaluation of medical devices — Part 1: Evaluation and testing
within a risk management process
•
ETS 300 019-2-1 Environmental Engineering (EE); Environmental conditions and environmental
tests for telecommunications equipment; Part 2-1: Specification of environmental tests; Storage
•
ETS 300 019-2-2 Environmental Engineering (EE); Environmental conditions and environmental
tests for telecommunications equipment; Part 2-2: Specification of environmental tests;
Transportation
•
ASTM D4169-16 Standard Practice for Performance Testing of Shipping Containers and Systems
for Vibration
•
IEC 60601-1:2005+A1:2012 - General Safety Ed. 3.1
•
IEC 60601-1-2:2014 – EMC Fourth Edition
•
IEC 60601-2-40:2016 – Particular requirements for the basic safety and essential performance of
electromyography and evoked response equipment
•
IEC 60601-1-6:2013 – Collateral Usability