14
Rev 13
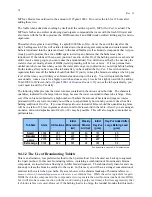
9.3.2 Total Alkalinity
Changes in pH can be caused by many factors and one significant cause is sanitizer use. Halogen sanitiz-
ers can have a significant impact on pH and water balance. However, changes in pH, due to such sanitizers
or shock, can be minimized by controlling the total alkalinity. This is a bit of a confusing term in the pure
chemical sense. Total Alkalinity refers to the ability of pool and spa water to resist changing in pH. A
product is added that essentially keeps the pH in the ideal range and it has reserve or pH holding capacity.
pH will drop only after the total alkalinity reserve is all used up. If you used distilled water at ph 7 and
just added a drop of acid, the pH would plunge. But with water that has a total alkalinity reserve, the pH
would only drop after sufficient quantity of acid was added to have neutralized (used up) the entire alka-
linity product. Total Alkalinity is adjusted upward using
Alka-Rise
(sodium bicarbonate) to a particular
ppm value, usually in the range of 80 -120 ppm. Usually an adjustment should not be made until total al-
kalinity is below 80 ppm. Downward adjustment requires an acid forming product, such as pH
Down
.
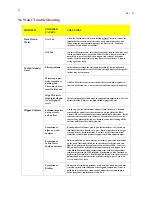
Water that is either too low or too high in total alkalinity will be out of balance and this will result in the
following consequences:
Total Alkalinity -
Normal 80-120ppm
High total alkalinity
Low total alkalinity
•
Hard to change pH
•
Scale formation
•
Cloudy water
•
Skin and eye irritation
•
Poor sanitizer efficiency
•
Poor ionizer operation
•
Rapid changes in pH or "pH bounce"
•
Corroded metals/equipment
•
Skin and eye irritation
9.3.3 Calcium Hardness
The term “hard water” originally came about because water with high levels of calcium does not clean
clothes well (hard to clean). The term hardness is now used only to refer to the level of calcium in the wa-
ter. Soft water refers to water with lesser or no amounts of contained calcium.
There is a balancing aspect to the amount of calcium desired in spa water. High levels tend to result in the
calcium wanting to precipitate out, especially at higher temperatures on heater surfaces (form scale) and
also at high pH or total alkalinity levels. Scale build up on heaters can lead to premature failure and in
decreased efficiency. Soft water on the other hand is aggressive and tends to attack metal parts such as
heat exchanges, heaters, heater tubes, etc. resulting in their premature destruction or in pinhole leaks. Well
water often is hard and lake or river water often is soft.
Some calcium is very desirable in spa water. The acceptable range of calcium in “balanced water” is 150 -
400ppm. There is no easy way to reduce water hardness except dilution with softer water. However, scal-
ing can be prevented by adding a sequestering agent, which ties up the calcium and prevents it from
precipitating. The product
Prevent II
that comes with our chemical kit, is such a sequestering product.
However, if you are using an ionizer, such a sequestering agent will also sequester to Cu, Ag and Zn metal
ions and prevent proper operation of the ionizer. In that case, either put up with the hard water or put the
fill water through a water softener. To increase calcium hardness calcium chloride is used. This is the
compound in the
Cal-Rise
container provided with in chemical kit.