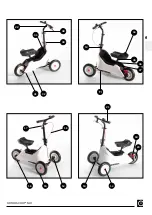
Quality assurance
All our auxiliary devices are delivered in good order and condition and are ready for use.
We constantly strive for improvements and process optimization in order to be able to prepare your
ORTHOSCOOT
®
for you as best as possible.
If, contrary to expectations, there should be a problem, please do not hesitate to contact us.
Declaration of liability
The company ORTHOSCOOT
®
GmbH acts as manufacturer. ORTHOSCOOT
®
GmbH shall only be liable if the ve-
hicle is properly and appropriately used as prescribed. ORTHOSCOOT
®
GmbH shall not be liable in case of impro-
per use (such as e.g. incorrect use, service or maintenance) or damages caused by com ponents or interferences
which ORTHOSCOOT
®
GmbH has not authorized.
Repairs may only to be carried out by ORTHOSCOOT
®
GmbH.
According to the provisions, the product has to be cleaned at regular intervals, however, on every change of pati-
ent!
Declaration of conformity
The company ORTHOSCOOT
®
GmbH, under its sole responsibility, declares conformity for the ORTHOSCOOT
®
NH1 series according to the European Medical Device Directive.
Scope of supply
1 ORTHOSCOOT
®
NH1
1 user manual
1 packaging board and transportation box
1 service log
1 user questionnaire
Explanation of components
01 Frame
02 Outer shell
03 Storage space
04 Knee rest
05 Pad knee rest
06 Clip knee rest
07 Locking pin knee rest
08 T-handlebar unit
09 Handlebar stem with tilting mechanism and clip
10 Control set
11 Steering linkage (tie rods and ball heads)
12 Steering knuckles
13 Axis
14 Quick release fastener for wheel
15 Front wheel
16 Rear wheel
17 Brake handle
18 Brake cable
19 Brake caliper
20 Brake disk
21 Handle
22 Bell
ORTHOSCOOT
®
NH1
32
Summary of Contents for NH1
Page 3: ...ORTHOSCOOT NH1 DE 01 ...
Page 9: ...22 03 08 09 02 01 10 04 05 06 07 17 18 16 15 12 13 14 21 19 20 ORTHOSCOOT NH1 DE 07 ...
Page 29: ...ORTHOSCOOT NH1 EN 27 ...
Page 35: ...22 03 08 09 02 01 10 04 05 06 07 17 18 16 15 12 13 14 21 19 20 ORTHOSCOOT NH1 EN 33 ...
Page 54: ...ORTHOSCOOT NH1 52 ...
Page 56: ......