5
ENGLISH
Medical Device
PRODUCT DESCRIPTION
The Icelock
®
544, referred to as the device in the following document,
consists of a variety of component configurations to support the use of
elevated and passive vacuum that help to provide and/or facilitate secure
coupling of the residual limb to the prosthesis specifically in non-weight-
bearing situations, i.e. swing phase during walking.
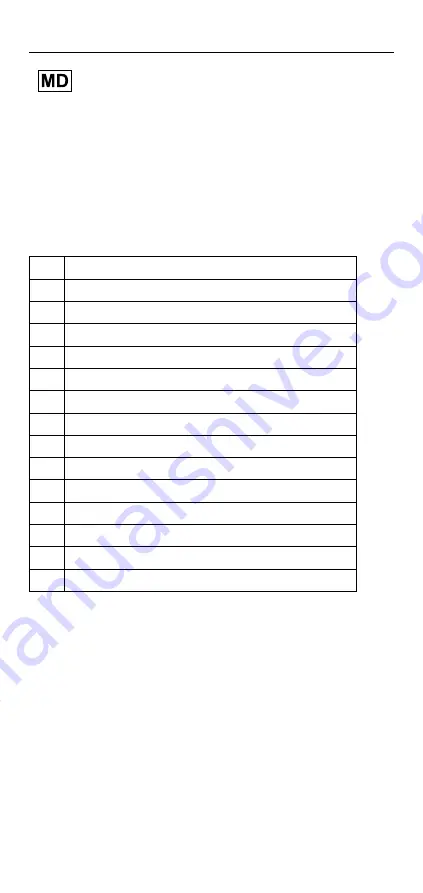
The different components can be configured as shown in Figure 1 and
the corresponding table. The names of the components are shown in the
following table.
A
Socket Adapter
B
Seal
C
Duckbill valve
D
Mesh Filter
E
Expulsion Housing
F
E2 Connector
G
Large Seal
H
O-Ring
I
Icelock
®
544 Expulsion Plate
J
Icelock
®
544 EP Plate
K
Icelock
®
544 Reservoir Plate / Icelock
®
544 Unity Plate
L
Icelock
®
544 LL Plate
M
LimbLogic Pump Seal
X
1
Housing dummy
X
2
Lamination dummy
INDICATIONS FOR USE
• Lower limb amputation and/or congenital deficiency
• No known contraindications
INTENDED USE
The device is intended to connect and release a prosthetic system that
replaces a missing lower limb.
The device contains a series of locks systems that can be configured in
different ways, making it compatible with many different suspension
systems.
The device is designed to facilitate suspension of a prosthesis,
specifically by:
• Creating differential air pressure in prosthetic socket;
• Permitting healthcare professional to locate and secure a locking
mechanism into socket during fabrication.






































