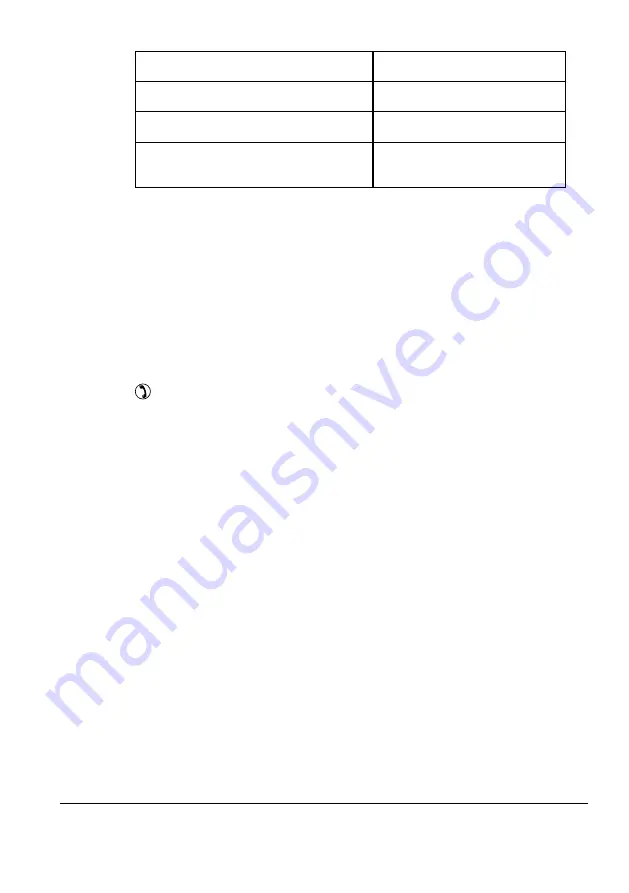
Contact pressure
8.8 N (± 0.5 N)
Weight with cable
approx. 490 g
Cable length
approx. 2.2 m
Standards
IEC 60645-1, IEC 60645-2
ANSI S3.6-2010
System-related technical specifications can be found in the user guide for your audi-
ometer.
10
Manufacturer
Natus Medical Denmark ApS
Hoerskaetten 9, 2630 Taastrup
Denmark
+45 45 75 55 55
www.natus.com
10.1
Responsibility of the manufacturer
The manufacturer is to be considered responsible for effects on safety, reliability, and
performance of the equipment only if:
•
All assembly operations, extensions, re-adjustments, modifications or repairs are
carried out by the equipment manufacturer or personnel authorized by the man-
ufacturer.
•
The electrical installation to which the equipment is connected complies with
EN/IEC requirements.
•
The equipment is used in accordance with the instructions for use.
The manufacturer reserves the right to disclaim all responsibility for the operating
safety, reliability and performance of equipment serviced or repaired by other
parties.
Otometrics - HDA 300
9
Summary of Contents for HDA 300
Page 12: ......






























