17
10 Cleaning and care
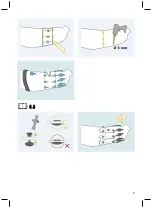
Cleaning the electrode domes
1) Clean the electrode domes with a cleaning cloth and 634A58 isopropyl alcohol after each
application.
2) Dry the electrode domes with a cloth.
11 Legal information
All legal conditions are subject to the respective national laws of the country of use and may vary
accordingly.
11.1 Liability
The manufacturer will only assume liability if the product is used in accordance with the descrip
tions and instructions provided in this document. The manufacturer will not assume liability for
damage caused by disregarding the information in this document, particularly due to improper
use or unauthorised modification of the product.
11.2 Trademarks
All product names mentioned in this document are subject without restriction to the respective
applicable trademark laws and are the property of the respective owners.
All brands, trade names or company names may be registered trademarks and are the property of
the respective owners.
Should trademarks used in this document fail to be explicitly identified as such, this does not justi
fy the conclusion that the denotation in question is free of third-party rights.
11.3 CE conformity
Otto Bock Healthcare Products GmbH hereby declares that the product is in compliance with
applicable European requirements for medical devices.
This product meets the requirements of the 2014/53/EU directive.
The product meets the requirements of the RoHS Directive 2011/65/EU on the restriction of the
use of certain hazardous substances in electrical and electronic devices.
The full text of the regulations and requirements is available at the following Internet address:
http://www.ottobock.com/conformity
11.4 Local Legal Information
Legal information that applies
exclusively
to specific countries is written in the official language
of the respective country of use in this chapter.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two
conditions:
1) This device may not cause harmful interference, and
2) This device must accept any interference received, including interference that may cause
undesired operation.
This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This equipment generates uses and can
radiate radio frequency energy and, if not installed and used in accordance with the instructions,
may cause harmful interference to radio communications. However, there is no guarantee that
interference will not occur in a particular installation. If this equipment does cause harmful inter
ference to radio or television reception, which can be determined by turning the equipment off