1 Foreword
English
INFORMATION
Date of last update: 2022-03-29
►
Please read this document carefully before using the product and observe the safety
notices.
►
Instruct the user in the safe use of the product.
►
Please contact the manufacturer if you have questions about the product or in case of prob
lems.
►
Report each serious incident related to the product to the manufacturer and to the relevant
authority in your country. This is particularly important when there is a decline in the health
state.
►
Please keep this document for your records.
The product "Myo Plus" is referred to as the product below.
These instructions for use provide you with important information on the use, adaptation and
handling of the product.
Only put the product into use in accordance with the information contained in the accompanying
documents supplied.
According to the manufacturer (Otto Bock Healthcare Products GmbH), the patient is the operat
or of the product according to the IEC 60601-1:2005/A1:2012 standard.
2 Product description
2.1 Function
The product is used to control a myoelectric prosthesis.
The product measures the patient's control signals and assigns them to the prosthesis move
ments.
By calibration via the Myo Plus app, the control unit learns to assign the sampled muscle signals
to the various movement types. This calibration can be carried out directly by the user and
repeated at regular intervals.
Essential performance of the product
•
No essential performance according to IEC 60601-1
2.2 Design
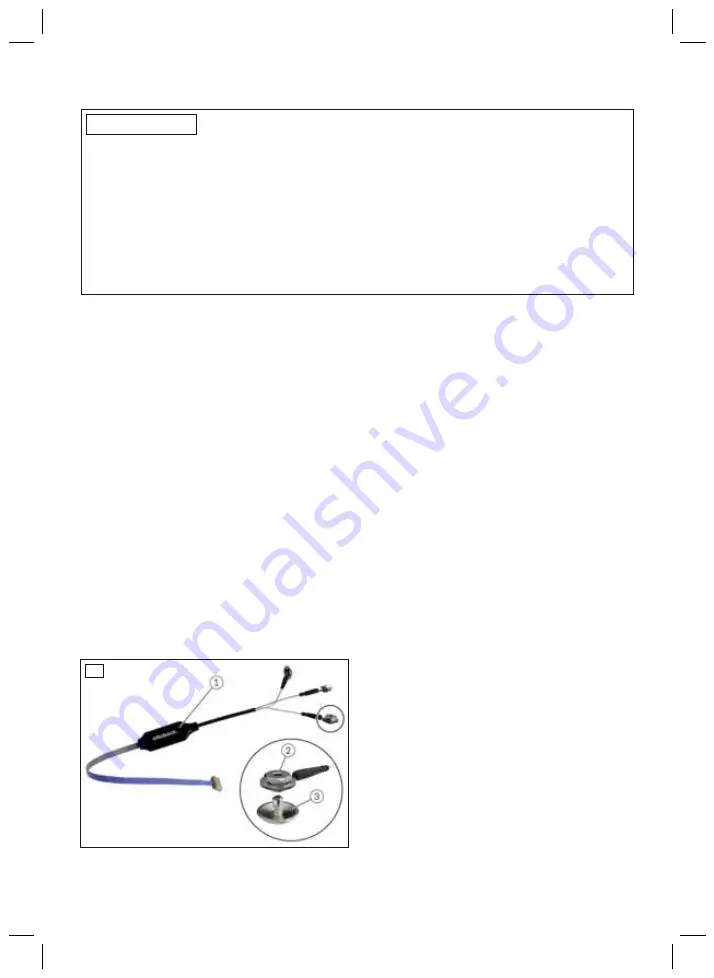
The product consists of the following components:
Remote electrodes
1
1. Electronics housing
2. Dome nut and cable lug
3. Electrode dome
5