Instructions for Use | Technical Specifications
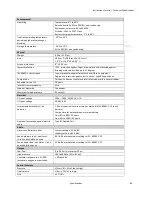
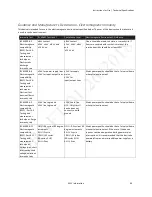
Standards Compliance
91
Standards Compliance
This device conforms to the following standards:
Standards
General
•
IEC 60601-1-1 Medical electrical equipment. Part 1-1: General requirements for
safety. Collateral standard: Safety requirements for medical electrical systems
Collateral
•
IEC 60601-1-11 Home Health Care Environment according to transit-operable
usage
Particular
•
Device essential performance is specified in each of the following standards:
•
ISO 80601-2-12: Medical electrical equipment. Part 2-12: Particular
requirements for basic safety and essential performance of critical care
ventilators
•
ISO 80601-2-61 Medical electrical equipment. Part 2-61: Particular
requirements for basic safety and essential performance of pulse oximeter
equipment
•
ISO 80601-2-55 Medical electrical equipment. Part 2-55: Particular
requirements for the basic safety and essential performance of respiratory gas
monitors
Wireless
communication
•
Bluetooth Core Specification version 4.1
•
ISO/IEC 18092:2013: Information technology. Telecommunications and
information exchange between systems. Near Field Communication. Interface
and
Protocol (NFCIP
-
1)
•
ISO IEC 21481 Ed 2.0: Information technology. Telecommunications and
information exchange between systems. Near Field Communication Interface
and Protocol -
2 (NFCIP
-
2)
•
ISO/IEC 14443 Ed 2.0: Identification cards. Contactless integrated circuit cards.
Proximity cards.
•
WLAN Standard:
IEEE 802.11 (2012)
b/g/n: Information technology.
Telecommunications and information exchange between systems. Local and
metropolitan area networks. Specific requirements. Part 11: Wireless LAN
Medium Access Con
trol (MAC) and Physical Layer (PHY) Specifications
•
Hereby, Respironics Inc. declares that this class 1 radio equipment is in
compliance with Directive 2014/53/EU. The full text of the EU declaration of
conformity is available at the following internet
address:
http://incenter.medical.philips.com/PMSPublic