16
TrueCPR Device Instructions for Use
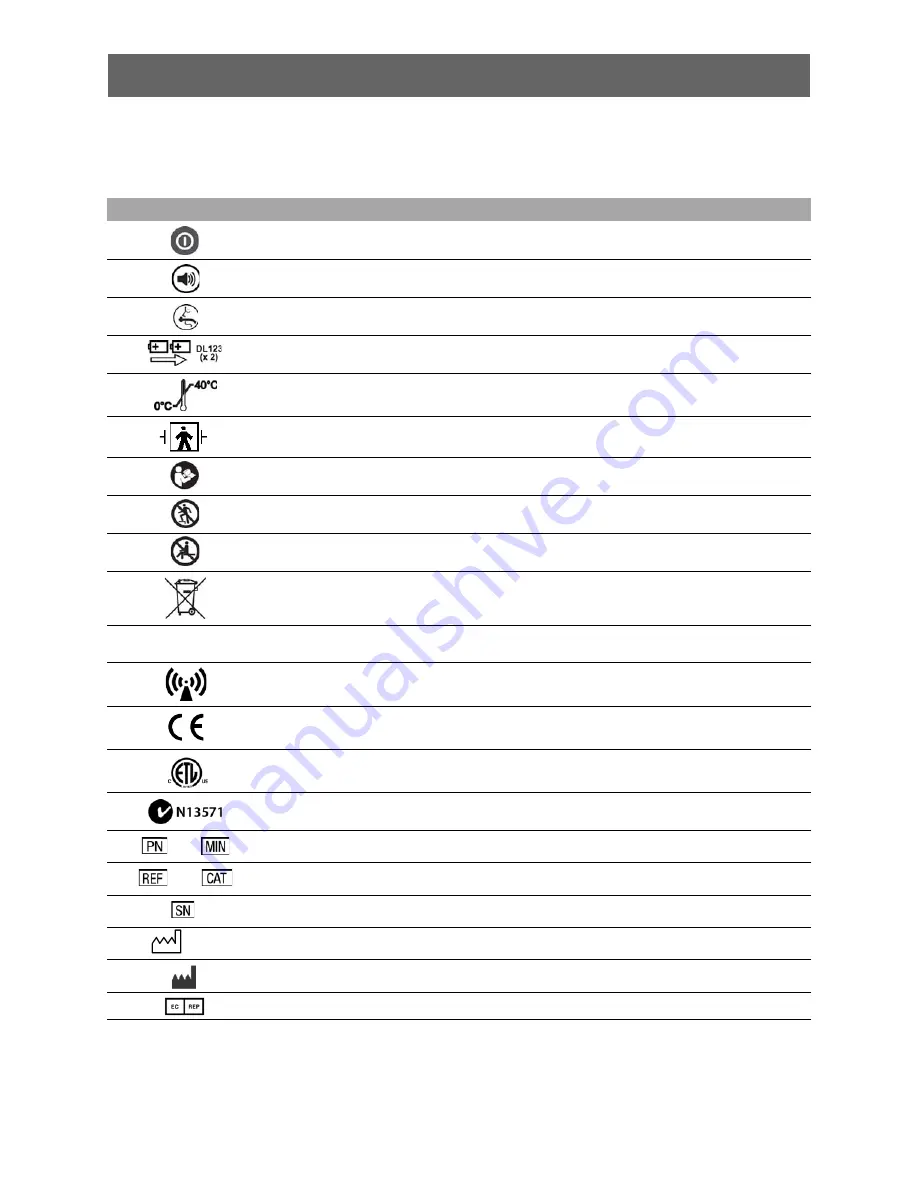
Symbols
Symbols
The following symbols may be found on the TrueCPR device or packaging.
SYMBOL
DESCRIPTION
Power button
Mute button
Airway button
Device requires two Duracell DL123 batteries (nonrechargeable)
Operating temperature 0° to 40°C (32° to 104°F)
Defibrillation proof, type BF applied part
Attention, consult accompanying documents. (Symbol has blue background and graphical
symbol is white.)
Do not step on the device.
Do not sit on the device.
Do not dispose of this product in the unsorted municipal waste stream. Dispose of this
product according to local regulations. For instructions on disposing of this product, see
www.physio-control.com/recycling.
Enclosure ingress protection code per IEC 60529. This rating provides for a specified
degree of protection against the ingress of particles and the harmful ingress of water.
Device includes RF transmitter.
Mark of conformity according to the European Medical Device
Directive 93/42/EEC
Mark of conformity to Canadian and US standards
Mark of conformity to ACA standards
Manufacturer’s identification number (part number)
Catalog number
Serial number
Date of manufacture
Manufacturer
Authorized EC representative
IP55
or
or
YYYY









































