46
The device has certificated to meet the following standards:
98/79/EC, IEC 60601-1, IEC 61010-1, IEC 60601-1-2, IEC 61326, and ISO 15197
IVD
CE
0123
2
LOT
!
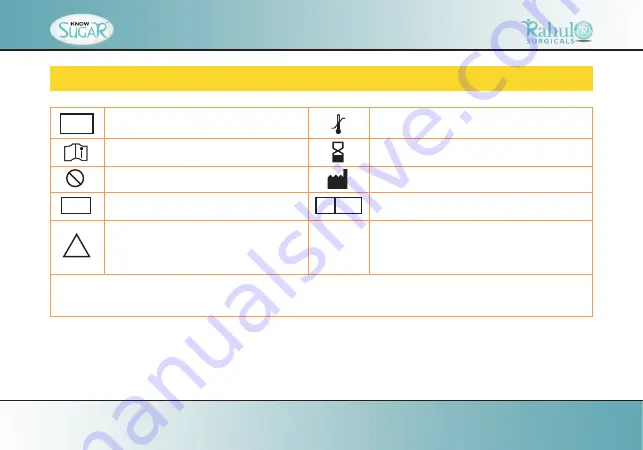
SYMBOLS DESCRIPTION
For in vitro diagnostic use.
Temperature limitation / Store at
Please consult instructions for use
Use by /Expiry date
Do not reuse
Manufacturer
Lot number
EU representative
Caution, consult
This product fulfils the requirements
accompanying document
of Directive 98/79/EC in vitro
diagnostic medical device.
EC REP
Summary of Contents for KNOW SUGAR
Page 50: ...50 FROM STAMP TO...























