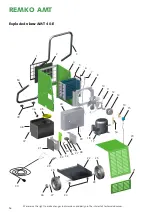
This shows that the rate of corro-
sion below 50% relative humidity
(R H) is insignificant and below
40% R H can be disregarded.
From 60% R H, the rate of corro-
sion increases considerably. This
moisture damage limit applies also
to numerous other materials, e.g.
powders, packaging, wood or elec-
tronic units.
Buildings can be dried out in differ-
ent ways:
1.
By heating and air exchange:
The room air is heated to absorb
moisture in order to then be dis-
charged to the atmosphere. The
total input energy is lost with
the discharged, moist air.
2.
By dehumidification:
The moist air in an enclosed
room is continuously dehumidi-
fied by the condensation princi-
ple.
The interrelated processes that
take place during dehumidification
are based on physical laws.
These are to be illustrated here in
simplified form in order to explain
the principle of dehumidification.
The use of
REMKO dehumidifiers
– No matter how well windows
and doors are insulated, damp
and moisture can penetrate
even through thick concrete
walls.
– The water volumes required for
binding concrete, mortar, plas-
ter, etc. are diffused out initially
after 1-2 months under certain
circumstances.
– Even moisture that has penetrat-
ed masonry following high wa-
ter or flooding, is only released
very slowly.
– This applies similarly, e.g. also to
moisture contained in deposited
materials.
The moisture (water vapour) es-
caping from buildings or materials
is absorbed by the ambient air. This
increases their moisture content
and ultimately results in corrosion,
mould, rot, peeling of paint coat-
ings and other unwanted moisture
damage.
The diagram opposite shows an
example of the rate of corrosion,
e.g. for metal at different humidity
levels.
Dehumidification
In terms of energy consumption,
dehumidification has one decisive
advantage:
Energy expenditure is restricted
solely to the existing room volume.
The mechanical heat released
through the dehumidification proc-
ess is returned to the room.
With correct use, the dehumidi-
fier only uses about 25% of the
energy that would be needed for
the „heating and ventilation”
principle.
The relative humidity
Our ambient air is a gas mixture
and contains always a certain
amount of water in the form of
water vapour. This water volume is
expressed in g per kg dry air (abso-
lute water content).
1m
air
weighs about 1,2 kg at 20 ° C
Depending on the temperature,
each kg of air is only able to ab-
sorb a certain amount of water va-
pour. When this absorptive capac-
ity is reached, reference is made to
“saturated” air; this has a relative
humidity of (R H) 100%.
Relative humidity is therefore un-
derstood to be the ratio between
the amount of water vapour cur-
rently contained in the air and the
maximum water vapour volume at
the same temperature.
The ability of air to absorb water
vapour increases with increas-
ing temperature. This means that
the maximum (= absolute) water
content increases with increasing
temperature.
rel. air humidity %
time of corr
osion
REMKO AMT