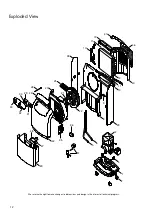
5
Water vapour condensation
The maximum possible amount of water vapour
that
can be absorbed becomes higher when the air is
heat-
ed
, but the water vapour
content remains unchanged
and consequently the relative air humidity is reduced.
However, when the air is
cooled,
the maximum possi-
ble amount of water vapour that can be absorbed is
continuously reduced although the water vapour quanti-
ty contained in the air remains unchanged and conse-
quently the relative air humidity rises.
When the air is further cooled, the absorption capacity
with respect to the maximum amount of water vapour
possible is continuously reduced until it is equivalent to
the water vapour content. This is the dew point temper-
ature.
If the air is cooled below the dew point, the water va-
pour content will be higher than the maximum amount
of water vapour possible.
W ater v a po ur is ex p e l l ed .
It condenses, is converted into water and thus extracted
from the air.
Steamed up window panes in winter or
steamed up bottles containing cold drinks
are typical examples of condensation.
The higher the relative air humidity is, the
higher the dew point temperature is and con-
sequently the easier it is for the tempera-
tures to fall below the dew point.
The resulting condensa-
tion is collected in the de-
vice and drained.
Condensation heat
The energy transferred from the condenser to the air
consists of:
◊
The heat that was previously extracted in the evapo-
rator.
◊
The electric operating power.
◊
The condensation heat released by liquefying water
vapour.
When the liquid is converted into a gaseous state, ener-
gy must flow back. This energy is called evaporation
heat. It does not cause the temperature to rise but is
used for the conversion from the liquid to the gaseous
state. Conversely, energy is released when gas is lique-
fied, and this energy is called condensation heat.
The energy produced from evaporation and condensa-
tion heat is identical.
For water this is 2250 kJ / kg ( 4.18 kJ = 1kcal )
This shows that a relatively high amount of energy is re-
leased through water vapour condensation.
If the humidity to be condensed is not generated by
evaporation within the room, but supplied from the out-
side, e.g. by ventilation, the condensation heat released
thereby contributes to heating the room.
When materials or rooms are to be dried, the heat ener-
gy flows in a cycle, i.e. it is consumed during evapora-
tion
and
released
during
condensation.
A larger amount of heat energy is generated when sup-
plied air is dehumidified which is expressed as a rise in
temperature.
Generally, the time needed for drying does not depend
on the unit capacity, but is determined by the speed at
which the material or the parts of the building release
the moisture contained in them.
The air current is cooled on its way through/via the
evaporator until its temperature falls below the dew
point. The water vapour condenses, is collected in a
condensation tray and drained.
Drying materials
Building materials/buildings can absorb considerable
amounts of water; e.g. bricks 90
-190 l/m³, heavy con-
crete 140-190 l/m³, calcareous sandstone 180-270 l/m³.
Damp materials such as brickwork dry as described be-
low:
◊
The moisture contained in the material
flows from the inside to the surface
◊
Evaporation takes place on the sur-
face = water vapour is absorbed by
the ambient air.
◊
The air augmented with water vapour continuously
circulates through the REMKO air dehumidifier. It is
dehumidified and leaves the unit in a slightly warm
state to absorb water vapour again.
◊
This allows the moisture contained in the material to
be continuously reduced; the material becomes dry.
°C
30
25
20
15
% r.F.
100
90
80
70
60
50
40
30
20
10
+
+
-
condenser
evaporator
air-temperature
air direction
humidity
time