5
Water Vapour Condensation
The maximum possible percentage of water vapour
that can be absorbed becomes higher while the air is
heated
, but the obtained percentage of water vapour
remains unchanged, and consequently the relative air
humidity is reduced.
However, when the air is
cooled
the maximum possi-
ble percentage of water vapour that can be absorbed
is continuously reduced, whereas the water vapour
quantity contained in the air remains unchanged, and
consequently the relative air humidity rises.
When the air is further cooled off the absorption ca-
pacity regarding the maximum possible water vapour
quantity is continuously reduced until it is equal to the
obtained percentage of water vapour. This is the dew
point temperature.
If the air is cooled down below the dew point, the ob-
tained percentage of water vapour will be higher than
the maximum possible water vapour quantity.
W a t e r v a p o u r i s d e p o s i t e d .
It condenses, is converted into water and thus is ex-
tracted from the air
Steamed up window panes in winter or
steamed up bottles containing cold drinks
are typical examples of condensation.
The higher the relative air humidity is, the
higher is the dew point temperature, and
consequently it is easier for the tempera-
tures to fall below the dew point.
The generated conden-
sate is collected in the
apparatus and carried
away.
Condensation Heat
The energy transferred from the condenser to the air
consists of:
◊
The heat that was previously extracted in the evapo-
rator.
◊
The electric driving energy.
◊
The condensation heat released by liquefying water
vapour.
When the liquid condition is converted into a gaseous
condition energy must flow back. This energy is called
evaporation heat. It does not cause the temperature to
raise but is used for the conversion from the liquid to
the gaseous state. On the other hand energy is re-
leased when gas is liquefied, and this energy is called
condensation heat.
The energy rate of evaporation and condensation heat
is identical.
For water this is 2250 kJ / kg ( 4,18 kJ = 1kcal )
This shows that a relatively high energy rate is released
through water vapour condensation.
If the humidity to be condensed is not generated by
evaporation within the room, but fed from the outside,
e.g. by aeration, the condensation heat released
thereby contributes to room heating.
When materials or rooms are to be dried the heat en-
ergy flows in a circulation, i.e. it is consumed during
evaporation and released during condensation.
A larger amount of heat energy is generated when fed
air is dehumidified, and this heat energy is expressed in
the form of a rise in temperature.
Generally the time needed for drying does not depend
on the apparatus capacity, but it is determined by the
speed at which the material or the parts of the building
emit the humidity contained in them.
The air current is cooled off on its way through/via the
evaporator until its temperature falls below the dew
point. The water vapour condenses, it is collected in a
condensate trap and carried away.
Material Drying
Building materials/buildings can absorb considerable
quantities of water; e.g. bricks 90-190 l/m³, heavy con-
crete 140-190 l/m³, calcareous sandstone 180-270 l/m³.
Humid materials such as brickwork dries in the following
way:
◊
The contained humidity flows from the
inside of the material to its surface
◊
Evaporation takes place on the sur-
face = water vapour is absorbed by the
ambient air.
◊
The air enriched with water vapour is continuously cir-
culating through the REMKO air dehumidifier. It is de-
humidified and leaves the apparatus in a slightly
heated up state to absorb again water vapour.
◊
By this the humidity contained in the material is con-
tinuously reduced; the material becomes dry.
°C
30
25
20
15
% r.F.
100
90
80
70
60
50
40
30
20
10
+
+
-
condenser
evaporator
air-temperature
air-direction
humidity
course
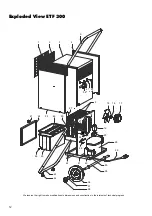
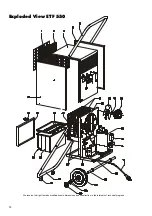
Summary of Contents for ETF 300
Page 2: ......