8-1
REM
staR
M s
ERiEs
U
sER
M
anUal
C
haptER
8: s
pECifiCations
E
nviRonMEntal
o
pERating
s
toRagE
t
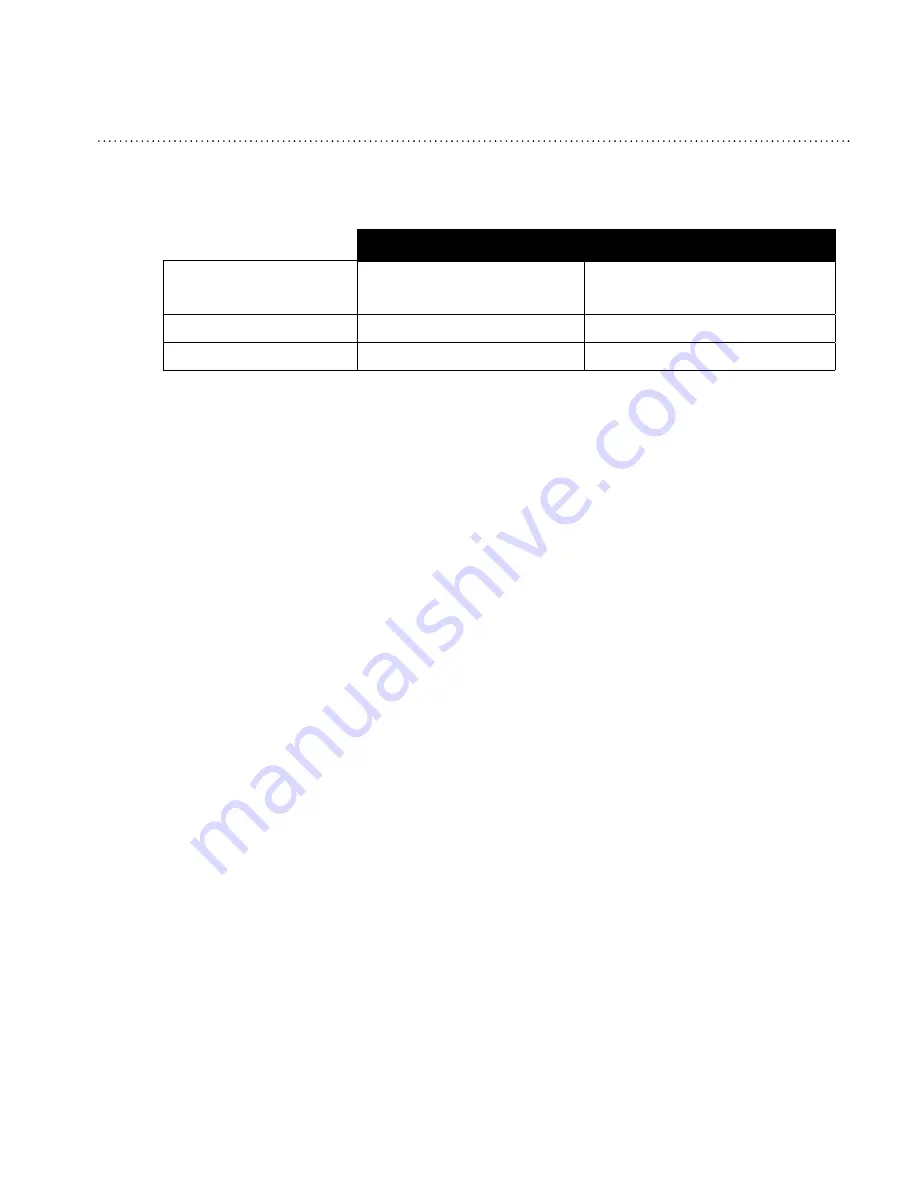
EMpERatURE
41° F to 95° F
(5° C to 35° C)
-4° F to 140° F
(-20° C to 60° C)
R
ElativE
h
UMidity
15 to 95% (non-condensing)
15 to 95% (non-condensing)
a
tMosphERiC
p
REssURE
77 to 101 kPa (0 - 7500 ft.)
N/A
p
hysiCal
Dimensions:
7.5 in. L x 5.0 in. W x 3.125 in. H (19 x 12.7 x 7.9 cm)
Weight:
Approximately 2.2 lbs. (1 kg) without a humidifier
s
tandaRds
C
oMplianCE
This device is designed to conform to the following standards:
– IEC 60601-1 General Requirements for Safety of Medical Electrical Equipment
– EN ISO 17510-1 Sleep Apnea Breathing Therapy Devices
E
lECtRiCal
AC Power Consumption:
100 – 240 VAC, 50/60 Hz, 1.0 A max.
DC Power Consumption:
12 VDC, 3.0 A max.
Type of Protection Against Electric Shock:
Class II Equipment
Degree of Protection Against Electric Shock: Type BF Applied Part
Degree of Protection Against Ingress of Water: Device: Drip Proof, IPX1
AC Power Supply: Drip Proof, IPX1
Mode of Operation:
Continuous
Electromagnetic Compatibility:
The device meets the requirements of
EN 60601-1-2, 2nd edition.
Fuses:
There are no user-replaceable fuses.