46
47
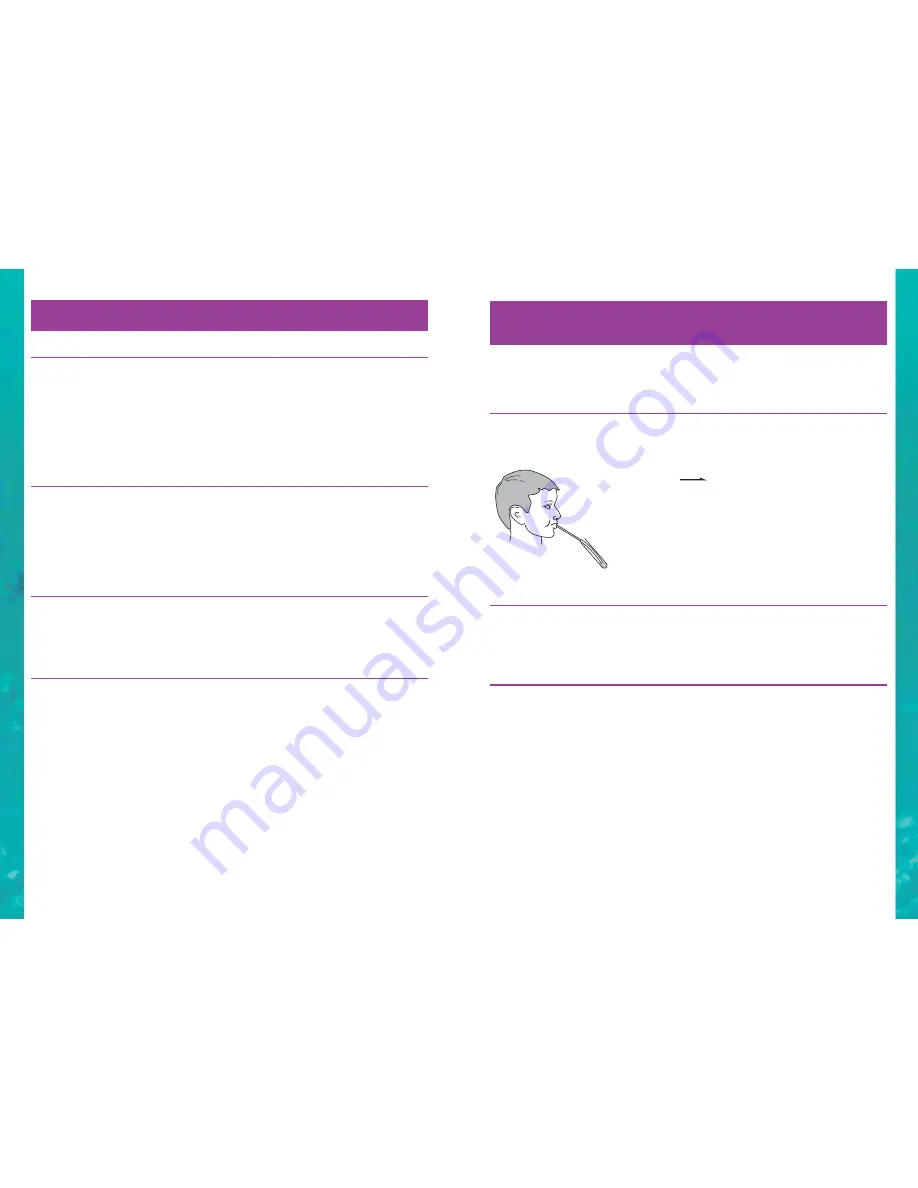
Experiment 9.1
To show that animals produce carbon dioxide
• lime water
• test tube
• glass & rubber tubing
Carbon dioxide can be detected by bubbling it through lime water
(calcium hydroxide solution). The clear lime water turns milky due
to the formation of the solid calcium carbonate.
The word equation for the reaction is: calcium hyd
carbon dioxide calcium carbonate.
Put 3cm of lime water in a test tube and gently blow through it for
a half a minute using the glass and rubber tubing. What happens?
Experiment 9.2
To show that air contains carbon dioxide
• lime water
• small glass
Put a little lime water into a small glass and leave it standing for a
day or so. Does the lime water turn slightly milky? What does this
show you?
Experiment 9.3
To show that carbon dioxide is an acid
• universal indicator paper
• test tube
• glass & rubber tubing
Carbon dioxide dissolves in water to give carbonic acid, as we saw
in Experiment 5.11 where universal indicator paper was used.
A more accurate test is to use universal indicator in solution. Fold
up 1 sheet of universal indicator paper and put it into a test tube.
Add 3cm of water. Gently shake to dissolve the indicator from the
paper, a green solution will be formed. Pour about half of this into
another test tube.
Blow gently through the indicator in the second test tube for about
1 minute using the glass and rubber tubing. Compare the colour
of the indicator with that left behind with the indicator paper. Is the
one you have blown into more yellow coloured? As you saw in
Chapter 5 acids turn universal indicator yellow then red. Carbon
dioxide has been shown to be an acid.
Experiment 8.6
Heating copper carbonate
• copper carbonate
• small evaporating spoon
In Experiment 7.1 you made a sample of copper carbonate.
Put the copper carbonate on the small metal evaporating spoon
and heat it over the burner flame. Describe what happens. What
colour is the residue in the spoon?
Look back at Experiment 6.7 to see if this contains any clues how
you might check what the residue is.
Experiment 8.7
Heating tartaric acid
• tartaric acid
• small evaporating spoon
Heat ½ measure of tartaric acid on the small evaporating spoon.
What happens? Are any gases evolved. Is there a residue after
heating for some time. (Tartaric acid contains carbon, hydrogen
and oxygen. The carbon forms carbon dioxide gas and the
hydrogen forms water vapour).
Experiment 8.8
Heating citric acid
• citric acid
• small evaporating spoon
Repeat experiment 8.7 using citric acid. Does it behave like
tartaric acid?
Experiment 8.9
Heating ammonium chloride
• ammonium chloride
• test tube
• beaker
Put 1 measure of ammonium chloride in a test tube and heat only
the bottom of the tube, gently at first and then more strongly.
Describe what happens. REMEMBER THE TUBE WILL BE HOT
- put it in an empty beaker until it is cool, not in the test tube rack.
Ammonium chloride is an unusual substance in that it
sublimes
.
This means that it changes from a solid straight into a gas or
gases without first becoming a liquid.
Chapter 9 - The chemistry of some gases
9a - Carbon dioxide
Chapter 8 - Heating substances
Carbon dioxide is an important gas. It is present in air and all animals produce it when breathing,
a process called
respiration
. It is used by plants during a process called
photosynthesis
when the
plants use the carbon dioxide as their source of carbon.











































