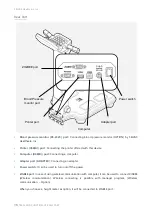
The device bears the CE label in accordance with the provisions of Medical Device Directive 93/42/EEC.
THE PERSONS RESPONSIBLE FOR PLACING DEVICES ON THE EC MARKET UNDER MDD 93/42/EEC
SELVAS Healthcare, Inc.
155, Sinseong-ro, Yuseong-gu, Daejeon, 34109 Republic of Korea
TEL: 82-42-879-3000, FAX: 82-42-864-4462
Obelis s.a.
Bd. Général Wahis 53.
B-1030 Brussels, Belgium.
Summary of Contents for ACCUNIQ BC300
Page 1: ...English 06 2021 V3 01 User Manual BC300...
Page 71: ......