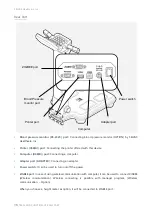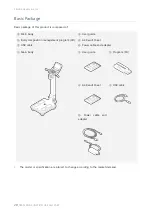
SELVAS Healthcare, Inc.
8
_INTRODUCTION
Note
This equipment has been tested and found to comply with the limits for medical devices
to the IEC 60601-1-2:2014. These limits are designed to provide reasonable protection
against harmful interference in a typical medical installation. This equipment generates
uses and can radiate radio frequency energy and, if not installed and used in accordance
with the instructions, may cause harmful interference to other devices in the vicinity.
However, there is no guarantee that interference will not occur in a particular installation.
If this equipment does cause harmful interference to other devices, which can be
determined by turning the equipment off and on, the user is encouraged to try to correct
the interference by one or more of the following measures:
Reorient or relocate the receiving device.
Increase the separation between the equipment.
Connect the equipment into an outlet on a circuit different from that to which the
other device(s) are connected.
Consult the manufacturer or field service technician for help.
Prohibition
Do not to touch signal input, signal output or other connectors, and the patient
simultaneously.
Note
A statement that MEDICAL ELECTRICAL EQUIPMENT needs special precautions regarding
EMC and needs to be installed and put into service according to the EMC information
provided in the ACCOMPANYING DOCUMENTS;
Note
A statement that portable and mobile RF communications equipment can affect MEDICAL
ELECTRICAL EQUIPMENT.
Note
Please consult a physician or a trained health professional for interpretation of
measurement results.
Caution
Measurements may be impaired if this device is used near televisions, microwave ovens,
X-ray equipment or other devices with strong electrical fields. To prevent such
interference, use the meter at a sufficient distance from such devices or turn them off.
Summary of Contents for ACCUNIQ BC300
Page 1: ...English 06 2021 V3 01 User Manual BC300...
Page 71: ......