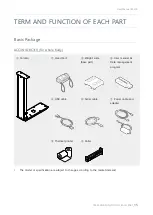
SELVAS Healthcare, Inc.
12
_INTRODUCTION
SYMBOL
INFORMATION
Medical Device
Guidance for Electromagnetic compatibility (EMC)
Details about the electromagnetic compatibility (EMC) of the ACCUNIQ BC310 are given below. Before
using the ACCUNIQ BC310, be sure to read and understand the following information.
1)
Guidance and manufacturer
’
s declaration – electromagnetic emissions
The ACCUNIQ BC310 is intended for use in the electromagnetic environment specified IEC 60601-1-
2:2014 (Fourth Edition).
2)
Guidance and manufacturer
’
s declaration – electromagnetic immunity
The ACCUNIQ BC310 is intended for use in the electromagnetic environment specified IEC 60601-1-
2:2014 (Fourth Edition).
3)
Guidance and manufacturer
’
s declaration – electromagnetic immunity 2
The ACCUNIQ BC310 is intended for use in the electromagnetic environment specified IEC 60601-1-
2:2014 (Fourth Edition).
4)
Recommended separation distances between portable and mobile RF communications equipment and
the ACCUNIQ BC310
The ACCUNIQ BC310 is intended for use in the electromagnetic environment specified IEC 60601-1-
2:2014 (Fourth Edition).