6
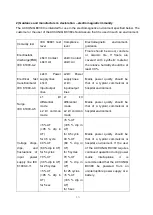
3. CLASSIFICATION AND COMPLIANCE
1) This device is classified as;
- Class 1 type-BF against electric shock
- Ordinary equipment without protection against ingress of water
- Equipment not suitable for use in presence of a flammable anesthetic mixture by standard of
EN 60601-1:2006/A1:2013(Basic safety and essential performance of Medical Electrical
Equipment)
2) This device is complied with Class A for Noise-Emission, Level B for Noise-immunity, by
standard of EN 60601-1-2:2007/AC:2010(Electromagnetic Compatibility Requirements).
4. SAFETY PRECAUTIONS
This device is designed and manufactured with consideration of the safety of the operator and
subject and also the reliability of the unit.
The following warnings, precautions and notes must be observed for safety;
Warning
During measurement of the body composition, a microcurrent of
180μA flows through
the body. Individuals who have any kind of implanted active medical devices, such as
pacemakers, should not use this equipment because the microcurrent can cause
malfunction in the implanted device.
Warning
To prevent fire hazard, use only a correctly wired (100-240VAC) outlet, and do not use
a MSO(Multiple Socket Outlet) with other device that is not in compliance with EN
60601-1.
Warning
To reduce the risk of electric shock or product damage, never plug-in or plug-out with
wet hands.
Warning
Physically disabled persons should not attempt to take measurements alone, but
instead should have their caretakers assist them in using the device.
Caution
The unit must be operated only by, or under supervision of a qualified person with our
company or our distributors.
Summary of Contents for Accuniq BC380
Page 1: ......