SEN 257
Issue 2.1
06 October 2020
Page
5
of
29
FCC mark
FCC mark is a certification mark employed on electronic products sold in the
United States which certifies that the electromagnetic interference from the
device is under limits approved by the Federal Communications Commission.
For prescription use only
Federal law in U.S. restricts this device to sale by or on the order of a
medical practitioner licenced by the law of the state in which he practices to
use or order the use of the device.
Unique Device Identification
The Unique Device Identification (UDI) is a system used to mark and identify
medical devices within the healthcare supply chain.
10. Getting Started
List for connecting to IntelliBridge
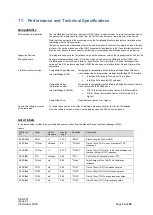
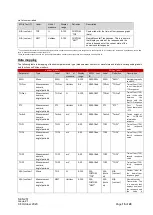
The following table identifies the equipment involved in the procedure.
Equipment
Part
number
TetraGraph Monitor
SEN 2001
TetraGraph Philips Interface SEN 2007
Operating Instructions
SEN 257
TetraGraph Monitor IFU
SEN 008
The TetraGraph monitor and TetraGraph Philips Interface are supplied by Senzime AB, the Philips IntelliBridge EC10 and
Philips IntelliVue monitors are supplied by Philips (Figure 2). IntelliBridge EC10 needs to have OpenInterface driver (option 101)
version A.6 or higher installed. No EC5 is required from Philips, since it is a part of SEN 2007. Philips part numbers for
IntelliBridge EC10:
IntelliBridge EC10 - Philips PN 865115 option A01,101
Figure 2
. Illustrating the products required for connecting the TetraGraph Monitor to Philips IntelliVue.