61 93 556 D3509
30
D3509
.
201.01.02
.
02
19.09.2008
3 System description
Sirona Dental Systems GmbH
External device connection
Operating Instructions TENEO
3.9
External device connection
External medical accessories can be connected to the external device
connection. They must comply with the requirements of Medical Device
Directive
93/42/EEC.
Connecting additional devices
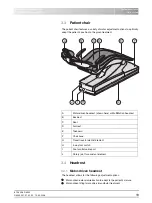
A
Swiveling cuspidor bowl
B
Tumbler filler (depicted) or tumbler filler with automatic sensor
control for automatic filling of the tumbler
C
Maintenance cover for disinfectant
D
Mount for support arm of operating light
E
Mount for tray support arm
F
Maintenance flap for accessing flushing valve, amalgam
separator, sediment container or filter insert for wet suction
CAUTION: Additional devices connected to the external device
connection are exposed to a hydrogen peroxide concentration of 0.1‰-
0.2‰.
If the additional devices are not suitable for the specified hydrogen peroxide
concentration, they may be damaged.
¾
Before connecting any additional devices, check to make sure that they
can be exposed to a hydrogen peroxide concentration. Contact the
manufacturer of the relevant additional device, if necessary.
¾
Additional devices must not be sanitized with the treatment center, see
"Sanitizing the treatment center" [
NOTE: DVGW approval
Due to the design of the treatment center according to EN 1717/DIN 1988
(DVGW requirements) the connected additional devices fulfill the above
standards.
NOTE: Self-contained power supply
The inlet connector remains live when the power switch is turned off. The
connected external devices must therefore possess their own power switch.
However, the air and water connections are switched off.