Clinical Study Information
Introduction
Heart failure (HF) is a major public health problem in the United States affecting over 5
million people with over 1 million HF hospitalizations per year. Elevated pulmonary artery
(PA) pressures may occur prior to signs and symptoms of HF decompensation and can
provide a sound physiologic basis for HF patient management.
The CardioMEMS HF System provides a novel method for measuring PA pressure using
a wireless pressure sensor implanted into the PA, an external communication device, and
a patient database. The CardioMEMS HF System provides physicians knowledge of PA
pressure while the patient is at home allowing them to manage patients proactively with
the goal of reducing heart failure hospitalizations.
The CHAMPION (CardioMEMS HF Sensor Allows Monitoring of Pressures to Improve
Outcomes in NYHA Functional Class III Heart Failure Patients) clinical trial was a
prospective, multicenter, single-blind, randomized, clinical trial in 550 patients across 64
centers in the United States.
Purpose
The goal of the CHAMPION clinical trial was to determine if physicians could reduce HF
hospitalizations by managing patient PA pressures using the CardioMEMS HF System.
Methods
Study Design
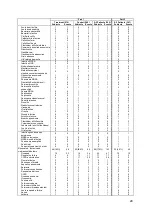
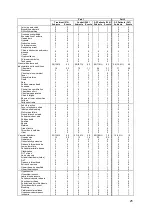
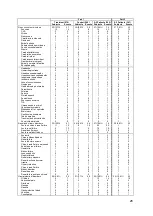
550 patients with NYHA Class III HF and a prior HF hospitalization within 12 months were
randomized to standard of care plus the CardioMEMS HF System (Treatment group; 270
patients) or to standard of care only (Control group; 280 patients). All patients were
implanted with the PA Sensor and took daily readings from home. Patients were enrolled
regardless of their baseline ejection fraction so that patients with both reduced and
preserved systolic function were included. Physicians had access to pulmonary artery
pressure information for patients in the Treatment group but not for patients in the Control
group.
Following the completion of Randomized Access, patients transitioned to a period of
Open Access, during which PA pressures were provided to physicians for patients in both
the Treatment and the Control groups. Specifically, physicians continued to have access
to the Treatment group’s PA pressures in an unchanged manner, whereas access to the
Control group’s PA pressure was provided for the first time.
Follow-Up Schedule
After right heart catheterization (RHC) and sensor implantation, all patients had follow-up
study visits at 1 month, 3 months, 6 months, and every 6 months thereafter until study
termination.
13