Also performed were an Anderson-Gill Model with Frailty, Anderson Gill Model with
Robust Sandwich Estimates (RSE) and Negative Binomial Regression using an endpoint
of time to HF hospitalization or death in Part 1 and Part 1 + Part 2. As seen in the gray
rows in Tables 11 and 12 below, all the competing risk analyses taking death into account
as a competing risk show that there was no evidence of a treatment-by-gender interaction
if a p-value of 0.05 is used. However, when analyses for interaction by gender are
conducted, a p-value of 0.15 is typically used because the analysis is typically not
powered appropriately. When considering a p-value of 0.15, there was some evidence of
treatment-by-gender interaction in the competing risk analyses under the following
models:
•
AG Model with Frailty for Part 1
•
NB Regression for Part 1
•
AG Model with Robust Sandwich Estimate for Part 1 + Part 2
•
GEE NB Regression for Part 1 + Part 2
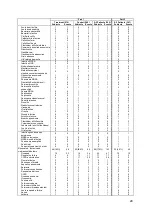
Table 11. Results of treatment by gender interaction using different statistical models
Models
Estimate
SE
p-value
Part 1
Cox Model: Endpoint of first HFR hospitalization or Death
-0.113
0.289
0.6968
Cox Model: Endpoint of first HFR hospitalization
-0.330
0.327
0.3131
AG Model with Frailty: Endpoint of HFR hospitalization or Death
-0.373
0.239
0.1211
AG Model with Frailty: Endpoint of HFR hospitalization
-0.531
0.262
0.0459
AG Model with RSE: Endpoint of HFR hospitalization or Death
-0.433
0.316
0.1712
AG Model with RSE: Endpoint of HFR hospitalization
-0.577
0.360
0.1094
NB Regression: Endpoint of HFR hospitalization or Death
-0.412
0.242
0.0896
NB Regression: Endpoint of HFR hospitalization
-0.573
0.191
0.0027
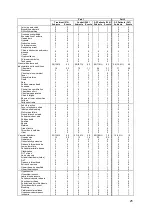
Part 1 + Part 2
Cox Model: Endpoint of first HFR hospitalization or Death
-0.204
0.249
0.4121
*
Cox Model: Endpoint of first HFR hospitalization
-0.427
0.284
0.1331
*
AG Model with Frailty: Endpoint of HFR hospitalization or Death
-0.376
0.274
0.1697
*
AG Model with Frailty: Endpoint of HFR hospitalization
-0.588
0.271
0.0301
*
AG Model with RSE: Endpoint of HFR hospitalization or Death
-0.477
0.274
0.0816
*
AG Model with RSE: Endpoint of HFR hospitalization
-0.642
0.313
0.0399
*
GEE NB Regression: Endpoint of HFR hospitalization or Death
-0.488
0.283
0.0841
*
GEE NB Regression: Endpoint of HFR hospitalization
-0.761
0.319
0.0172
*
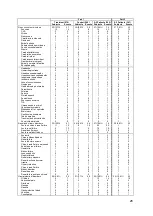
Table 12. The Treatment vs. Control effects by Gender over Part 1 and over Part 1+ Part 2 under
different models
Males
Hazard Ratio p-value
Part 1 (Treatment vs. Control)
AG Model with Frailty: Endpoint of HFR hospitalization or Death
0.67
0.0007
AG Model with Frailty: Endpoint of HFR hospitalization
0.64
0.0004
Part 1 + Part 2 (Former Control vs. Control)
AG Model with Frailty: Endpoint of HFR hospitalization or Death
0.70
0.0176
*
AG Model with Frailty: Endpoint of HFR hospitalization
0.53
<0.0001
*
Females
Hazard Ratio p-value
Part 1 (Treatment vs. Control)
AG Model with Frailty: Endpoint of HFR hospitalization or Death
0.99
0.9440
AG Model with Frailty: Endpoint of HFR hospitalization
1.07
0.7584
Part 1 + Part 2 (Former Control vs. Control)
AG Model with Frailty: Endpoint of HFR hospitalization or Death
0.80
0.4512
*
AG Model with Frailty: Endpoint of HFR hospitalization
0.61
0.1482
*
(
*
P-values should be interpreted with caution because the analyses including Part 2 data were not specified before
the onset of the study and there are various sources of confounding effects which cannot be separated from the
treatment effect.)
20