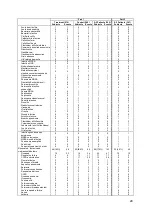
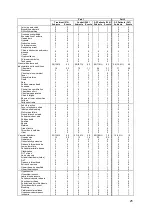
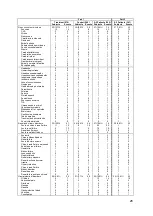
Unanticipated Serious Adverse Device Events
There was one event during Part 1 reported as a USADE by the investigator but
determined not to be serious or device system related by the CEC which reviewed the
event on 27 Jun 2009. There were no additional USADEs over Part 2 of the clinical trial.
Serious Adverse Device Events
The two SADEs that occurred during Part 1 were hemoptysis during the implant
procedure and an in-situ thrombosis during the right heart catheterization procedure.
Both patients were treated and recovered without sequela. There were no additional
SADEs over Part 2 of the clinical trial.
FCC Statement
This device is approved for wireless transmission under FCC ID number R3PCS-A-
000051. This device complies with Part 15 of the FCC Rules. Operation is subject to the
following conditions:
•
This device may not cause harmful interference.
•
This device must accept any interference received, including interference that may
cause undesired operation.
Technical Support
For technical support, call 1 877 696 3754.
31