8
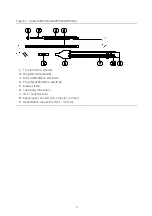
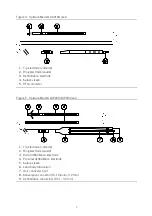
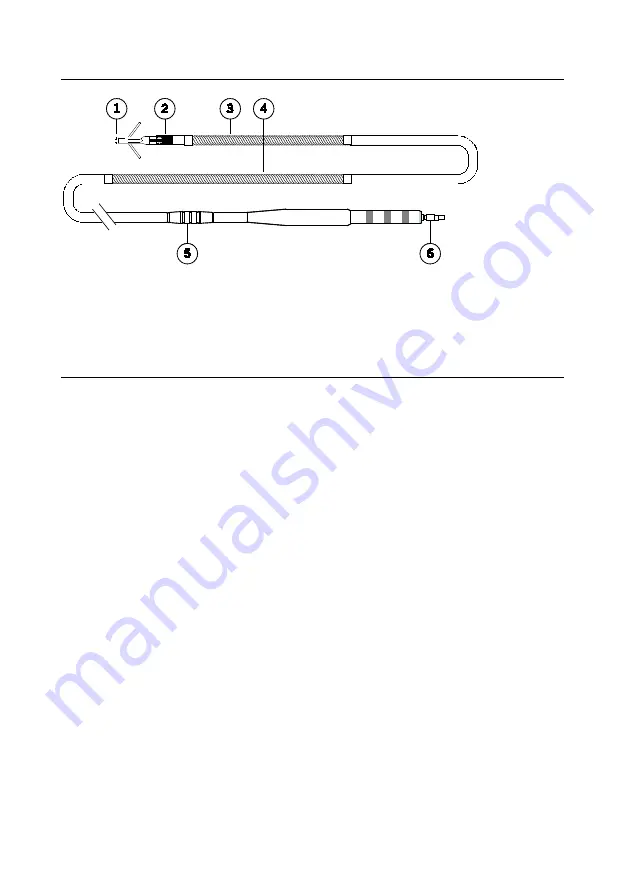
Figure 6. Optisure Models LDP220Q/LDP230Q lead
1.
Tip electrode (cathode)
2.
Ring electrode (anode)
3.
Distal defibrillation electrode
4.
Proximal defibrillation electrode
5.
Suture sleeve
6.
DF4 connector
Indications
The Optisure
™
Models LDA220, LDA230, LDA210, LDA220Q, LDA230Q, LDA210Q, LDP220,
LDP230, LDP220Q, and LDP230Q transvenous leads are indicated for use with compatible pulse
generators (refer to the applicable defibrillator manual for system indications). They provide
pacing and sensing and deliver cardioversion/defibrillation therapy to the heart.
Contraindications
Optisure
™
Models LDA220, LDA230, LDA210, LDA220Q, LDA230Q, LDA210Q, LDP220,
LDP230, LDP220Q, and LDP230Q leads are contraindicated in the following:
Patients with tricuspid valvular disease or a mechanical tricuspid valve.
Patients with ventricular tachyarrhythmias resulting from transient or correctable factors
such as drug toxicity, electrolyte imbalance, or acute myocardial infarction.
Patients for whom a single dose of 1.0 mg of dexamethasone sodium phosphate is
contraindicated.
For use with extra firm (red color knob) stylets (models LDA220, LDA230, LDA210,
LDA220Q, LDA230Q, and LDA210Q).
Warning
Implanted cardiac leads are subjected to a hostile environment within the body due to
constant, complex flexural and torsional forces, interactions with leads and/or the pulse
generator, or other forces associated with cardiac contractions and patient physical activity,
posture, and anatomical influences. Cardiac leads' functional lifetimes can be affected by
these and other factors.
Use only battery-powered equipment when implanting and testing the lead to avoid