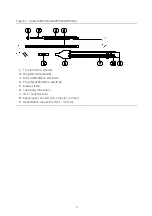
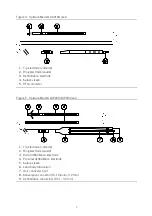
12
Table 5. Potential Adverse Events
Event
Possible Effects
skin erosion, tricuspid valve dysfunction, chronic
mechanical stimulation of the heart
Contamination
Infection requiring removal of lead system, pulse
generator, or both
Post-shock rhythm disturbances
Post-shock bradycardia or supraventricular
arrhythmias, conduction disturbances
Threshold elevation or exit block
Loss of efficacy of defibrillation, cardioversion, or
pacing therapy
Shunting or insulating of current during
defibrillation with internal or external
paddles
Increased external defibrillation energy and/or
repositioning of paddles required
Clinical Studies in the Durata™ Models 7120Q, 7121Q,
7122Q, 7170Q Leads
The Optisure
™
leads are similar to the Durata
™
leads except for increased Optim
™
insulation
thickness in the proximal region of the lead and the addition of Optim
™
insulation underneath the
SVC defibrillation coil. The clinical data presented in this document were collected on the Durata
™
7120Q, 7121Q, 7122Q, and 7170Q lead models that are part of a clinical study (SJ4 Post
Approval Study). The purpose of the clinical study, which included Durata
™
7120Q, 7121Q,
7122Q and 7170Q lead models, was to evaluate the safety and effectiveness of the SJ4 system
(device with DF4 connector and RV high voltage SJ4 leads). SJ4 is equivalent to DF4. The Q-
version of the Durata
™
lead models are identical to the non-Q version except for the type of
connector.
Patients Studied
As of the 3 years interim report (data cut-off date–June 29, 2012), 1697 patients were implanted
with the SJ4 system in the clinical trial. Patient enrollment began on June 4, 2009 and was
completed on July 13, 2010. Patients in this study will have a 5 year follow-up period from
implant. Refer to the following table for patient follow-up status as of the interim report. The
population was predominantly male (71.7%) with a mean age of 65 years.
Table 6. Patient follow-up status
Visit Type
Expected at this
Visit (N)
Followed
Through
(Showed Up) at
this Visit % (N)
Withdrawals Not
Due to
Unsuccessful
Implants or
Deaths
Not Yet Due at
this Visit (N)
Implant
1697
100.0% (1697)
0
0
6 month
1591
93.1% (1521)
42
0
12 month
1497
91.1% (1432)
75
0
7
The denominator is the total number of expected visits plus withdrawals not due to death or unsuccessful implants.