26
Protege IPG Clinician’s Manual
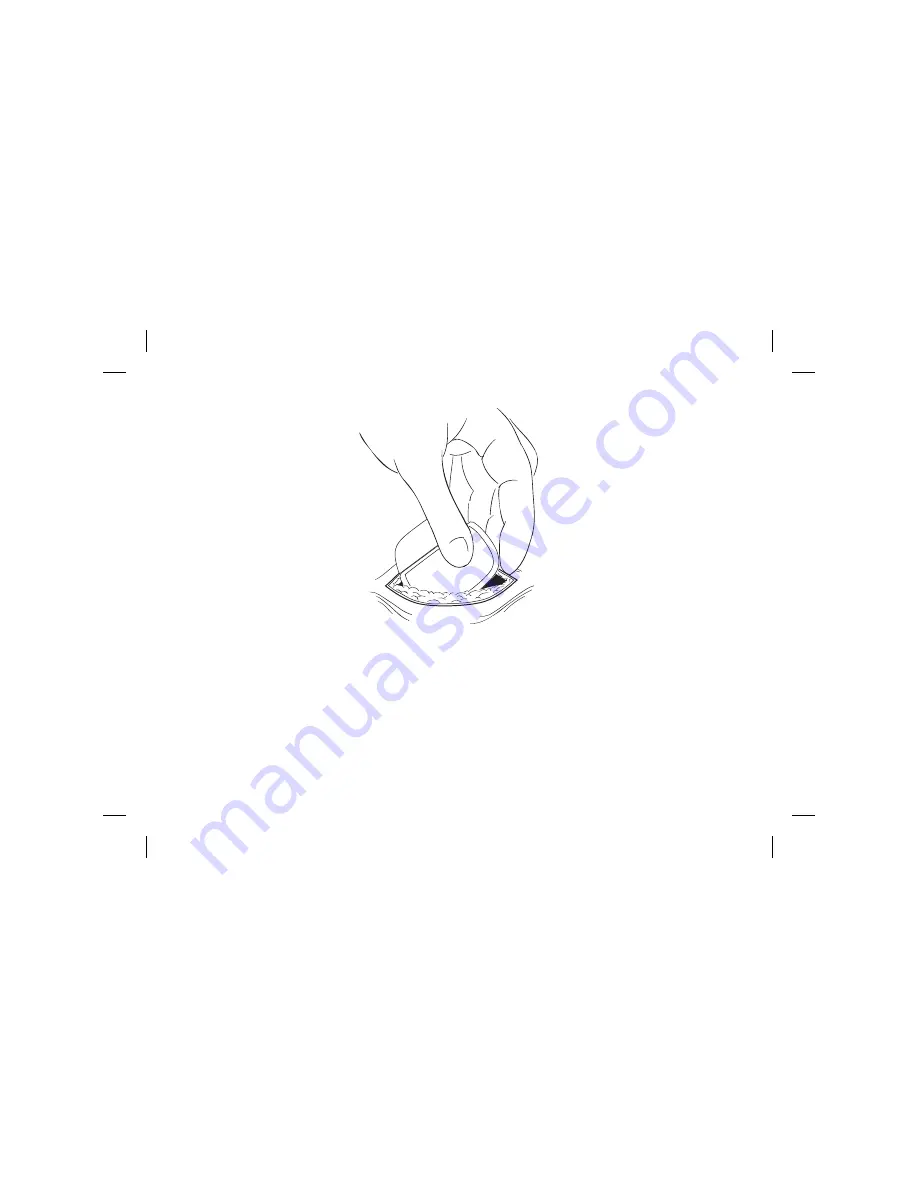
Figure 7: Place the IPG in the pocket
2. Carefully coil any excess lead or extension behind the IPG in loops no
smaller than 2.5 cm (1 in) in diameter to provide strain relief for the lead or
extension and IPG connection.
37-4832-01A.indd 26
37-4832-01A.indd 26
1/30/2014 4:39:29 PM
1/30/2014 4:39:29 PM
















































