INSTRUCTIONS FOR USE
INTENDED USE
CD-Chex CD34
®
is a stabilized preparation of human blood to be used as a complete process control
when evaluating CD34 positive cells. It is intended to be used with BD Biosciences ProCOUNT™
Progenitor Cell Enumeration Kit, BD™ Stem Cell Enumeration Kit, Beckman Coulter
®
Stem-Kit™, and
with systems using the ISHAGE protocol for enumeration of CD34 cells.
SUMMARY AND PRINCIPLES
CD34 enumeration by flow cytometry provides a rapid and accurate assessment of the frequency of
CD34 positive progenitor cells in samples from bone marrow, cord blood or peripheral blood from
patients treated with hematopoietic growth factors. The ability to quantitate CD34 cells is useful in
hematopoietic transplantation.
CD34 positive progenitor cells can be distinguished on the basis of light scatter properties in
conjunction with surface antigens. CD-Chex CD34 is designed to represent a blood sample
containing CD34 positive cells having characteristics similar to progenitor cells: low/intermediate
side scatter properties, CD34 expression and low expression of CD45 (compared to lymphocytes).
1,2,3
When stained with monoclonal antibodies for CD34 positive cell enumeration, CD-Chex CD34 control
will provide reference values for CD34 positive cells within the ranges on the assay sheet.
REAGENTS
CD-Chex CD34 contains human leukocytes and erythrocytes in a preservative medium.
PRECAUTIONS
1. For In Vitro Diagnostic Use.
2. CAUTION: All blood products should be treated as potentially infectious. Source material from
which this product was derived was found negative when tested in accordance with current FDA
required tests. No known test methods can offer assurance that products derived from human
blood will not transmit infectious agents. See the Instructions (IFU) tab under Resources on the
product page at www.streck.com for specific FDA required blood tests.
3. This product should not be disposed in general waste, but should be disposed with infectious
medical waste. Disposal by incineration is recommended.
4. This product is intended for use as supplied. Adulteration by dilution or addition of any materials
to the product vial invalidates any diagnostic use of the product.
5. CD-Chex CD34 products should not be used as a calibrator.
6. SDS can be obtained at www.streck.com, by calling 800-843-0912, or by calling your local
supplier.
STORAGE AND STABILITY
CD-Chex CD34 is stable through the expiration date when stored at 2 °C to 10 °C. After opening,
CD-Chex CD34 is stable throughout the open-vial dating, as indicated on the assay sheet, when
stored at 2 °C to 10 °C. DO NOT FREEZE.
INDICATIONS OF PRODUCT DETERIORATION
If CD-Chex CD34 values are not within the expected range on the assay sheet or granulocytes show
a loss of forward scatter (FSC):
1. Review control product package insert and the operating procedure of the instrument.
2. Check expiration date of the product on the vial. Discard outdated products.
3. Assay an unopened vial of CD-Chex CD34. If the values are still outside the Expected Range,
contact Streck Technical Services at 800-843-0912 or technicalservices@streck.com.
4. Clumping of the cell suspension indicates instability or deterioration, in which case the product should
not be used.
INSTRUCTIONS FOR USE
CD-Chex CD34 is designed to be used with ISHAGE CD34 enumeration protocols.
1,2,3
1. Follow instrument manufacturer’s instructions for instrument alignment and sample analysis.
2. Remove a vial of the control from refrigerator and warm to room temperature (18 °C to 30 °C) for
15 minutes before use.
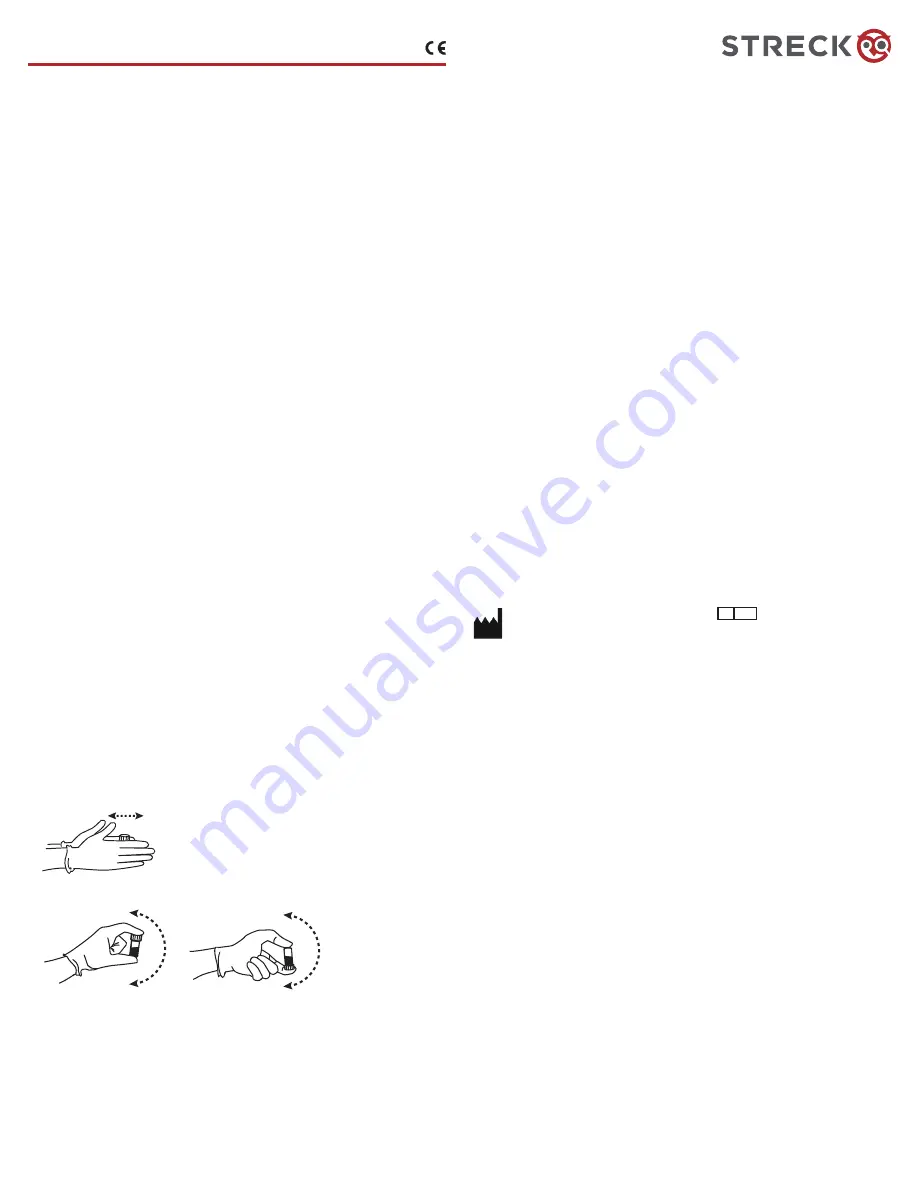
3. Mixing Procedure
(mechanical mixing by vortex or rotator is not recommended)
:
For a video demonstration, visit www.streck.com/mixing.
a. Holding the vial vertically between the palms of the hands, roll the vial back and forth for
20-30 seconds.
b. Hold the vial by the ends between the thumb and finger, and mix by gently inverting the vial
at least 8-10 times end-over-end until all cells are thoroughly suspended.
c. Aliquot immediately after mixing.
d. Subsequent analyses during this test period may be performed by inverting the vial 5 times
prior to sampling.
Note: Vials stored for an extended period of time may require extra mixing.
4. Return control reagent to refrigeration after sampling to ensure maximum open-vial stability.
5. Add recommended monoclonal antibodies according to manufacturer’s instructions to each tube
and mix gently.
6. Incubate according to antibody manufacturer’s instructions.
7. Add recommended amount of RBC lysing agent and follow manufacturer’s instructions.
8. Analyze by flow cytometry using your laboratory’s established protocol.
LIMITATIONS
1. CD-Chex CD34 is not recommended for use with antibodies that target the class I and class II
epitopes of CD34.
2. CD-Chex CD34 is designed to be used with a RBC lysing agent and may not provide results within
the assay range if analyzed without RBC lysis.
EXPECTED RESULTS
The mean assay values provided for each parameter are derived from replicate analyses on properly
compensated flow cytometers.
1,2,3,4
The assay values are obtained using common flow cytometry
reagents. See the IFU and assay for limitations or specific instructions for reagents.
Upon receipt of a new control lot, it is recommended that an individual laboratory establish its
own mean and limits for each parameter. However, the control means established by the laboratory
should fall within the expected range specified for the control. The expected ranges listed represent
estimates of variation due to different reagents, laboratory protocols, instrument calibration,
maintenance, and operator technique.
REFERENCES
1. Clinical and Laboratory Standards Institute, H42-A2, Enumeration of immunologically defined cell
populations by flow cytometry. Approved Guideline - Second Edition.
2. Keeney M, Chin-Yee I, Weir K, Popma J, Nayar R, Sutherland DR. Single platform flow cytometric
absolute CD34+ cell counts based on ISHAGE guidelines. Cytometry (Communications in Clinical
Cytometry) 1998; 34:61-70.
3. Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee IH. The ISHAGE guidelines for CD34+
cell determination by flow cytometry. Journal of Hematotherapy 1996; 5:213-226.
4. Marti G, Johnsen H, Sutherland R, Serke S. Letter to the editor: A convergence of methods for a
worldwide standard for CD34+ cell enumeration. Journal of Hematotherapy 1998; 7:105-109.
QUALITY CONTROL PROGRAM
Streck offers
STATS
®
, an interlaboratory quality control program, to all customers at
no charge. For more information, contact the
STATS
Department at 800-898-9563 or
statsdata@streck.com. Additional information can be found at www.streck.com.
ORDERING INFORMATION
Please call our Customer Service Department at 800-228-6090 for assistance. Additional information
can be found online at www.streck.com.
GLOSSARY OF SYMBOLS
See the Instructions (IFU) tab under Resources on the product page at www.streck.com.
The brand and product names of the instruments are trademarks of their respective holders.
See www.streck.com/patents for patents that may be applicable to this product.
350421-24
2018-04
Streck
7002 S. 109 Street, La Vista, NE 68128 USA
M
EDI
M
ARK
®
Europe
11, rue Emile Zola, BP 2332
38033 Grenoble Cedex 2, France
EC REP
CD-Chex CD34
®




























