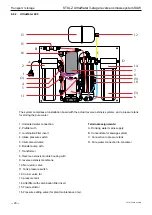
Transport / storage
STULZ UltraWater 3-stage reverse osmosis system SUW
© STULZ GmbH, Hamburg
- 14 -
If two solutions with different ion concentrations – aqueous solutions with different salt content – are sepa
-
rated by a semi-permeable membrane, the solutions attempt to equalise the concentration. This attempt
results in ions from the aqueous solution, with the higher concentration trying to “migrate” to the side of the
semi-permeable membrane the lower concentration solution.
This concentration equalisation cannot occur in this way, as the membrane is impermeable for the ions.
Thus, the water molecules, which can penetrate the membrane, flow from the side with the lower concen
-
trate to the side with the higher concentration to achieve the equalisation. This process continues until
the concentration equalisation of the ions on both sides has been achieved or so-called osmotic pressure
has become established on the high concentration side. This osmotic pressure is less than 2 bar in drink
-
ing water and increases as the different in concentration rises. This process is known as osmosis and, in
nature, is responsible for regulating the water balance in cells.
The bursting of cherries is described below as a clear example of osmosis.
The starting point is a ripe cherry, whose skin is wet with water after a rain shower.
The rain water on the skin of the cherry represents a liquid with lower concentration of dissolved particles.
The cherry juice inside the cherries represents a liquid with high concentration due to the dissolved sugar.
The cherry juice attempts to equalise the concentration with the rain water and wants to diffuse towards
this water. However, this is prevented by the semi-permeable membrane, which retains the sugar mol
-
ecules because of their size.
This results in a process in which the water molecules flow from outside into the inside of the cherry and
the pressure in the cherry increases until it can no longer expand and finally bursts.
As the name suggests, reverse osmosis is the converse of the osmosis process.
Tap water represents the side with high ion concentration and is forced towards the pure water with the low
concentration by application of an external pressure.
This externally applied pressure must be higher than the osmotic pressure. This is the only way in which
the water molecules can migrate against their natural osmotic direction into the area with lower concentra
-
tion and the substances dissolved in the tap water cannot pass through the semi-permeable membrane
because of their size. Therefore, almost exclusively water molecules remain on the pure water side. The
substances remaining on the membrane must be continuously discharged to prevent clogging of the mem
-
brane.
-
5
-
© STULZ GmbH, Hamburg
-
5
-
Reverse osmosis technology
If two solutions with different ion concentrations – aqueous solutions with different salt content – are separate
by a semi-permeable membrane, the solutions attempt to equalise the concentration. This attempt results in
ions from the aqueous solution with the higher concentration trying to “migrate” to the side of the semi-perme
-
able membrane with the lower concentration solution.
This concentration equalisation cannot occur in this way, as the membrane is impermeable for the ions. Thus,
the water molecules, which can penetrate the membrane, flow from the side with the lower concentrate to the
side with the higher concentration to achieve the equalisation. This process continues until the concentration
equalisation of the ions on both sides has been achieved or so-called osmotic pressure has become estab
-
lished on the high concentration side. This osmotic pressure is less than 2 bar in drinking water and increases
as the different in concentration rises. This process is known as osmosis and, in nature, is responsible for
regulating the water balance in cells.
The bursting of cherries is described below as a clear example of osmosis.
The starting point is a ripe cherry, whose skin is wet with water after a rain shower.
The rain water on the skin of the cherry represents a liquid with lower concentration of dissolved particles. The
cherry juice inside the cherries represents a liquid with high concentration due to the dissolved sugar.
The cherry juice attempts to equalise the concentration with the rain water and wants to diffuse towards this
water. However, this is prevented by the semi-permeable membrane, which retains the sugar molecules be
-
cause of their size.
This results in a process in which the water molecules flow from outside into the inside of the cherry and the
pressure in the cherry increases until it can no longer expand and finally bursts.
As the name suggests, reverse osmosis is the converse of the osmosis process.
Tap water represents the side with high ion concentration and is forced towards the pure water with the low
concentration by application of an external pressure.
This externally applied pressure must be higher than the osmotic pressure. This is the only way in which the
water molecules can migrate against their natural osmotic direction into the area with lower concentration and
the substances dissolved in the tap water cannot pass through the semi-permeable membrane because of
their size. Therefore, almost exclusively water molecules remain on the pure water side. The substances re
-
maining on the membrane must be continuously discharged to prevent clogging of the membrane.
Osmosis principle
Reverse osmosis principle
High concentration
Low concentration
High concentration
Low concentration
Direction of flow
External pressure
Direction of flow
Mem
bran
e
Membran
e
6.1. The principle of the reverse osmosis system