8
ENGLISH
PRODUCT DESCRIPTION
Classification of medical devices pursuant to Directive 93/42/EEC (Annex IX)
-
Duration: Temporary
-
Invasive devices: Non-invasive
-
Active medical device: Active
Due to the above characteristics the device falls under class I (in accordance with
Rule 1)
Classification in accordance with general regulations (standard CEI EN 60601)
-
According to the type of protection against electrical hazards: Class I equipment
-
According to the type of protection against ingress of water: Ordinary equipment
-
According to the degree of safety in use in the presence of a flammable anaesthetic mixture
with air or oxygen or nitrous oxide: Device not suitable for use with what is described
above.
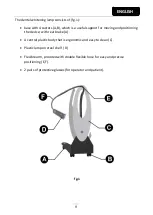
The dental whitening lamp is for use in the odontoiatry.
Dental whitening is the only and sole purpose of device
The lamp emits an intense light radiation.
The operator who uses the device must have the necessary
qualifications and
experience.
The operator must have received the necessary training in its correct use and must
know the position of all the controls:
•
for positioning the lamp
•
for switching the lamp on and off.
Read the instructions for use carefully before installing and using
the device.
Illegitimate use of the device and operator negligence can cause
damage to persons, the environment and property; the
manufacturer cannot be held responsible for said events.
The operator who uses the device must have been correctly
trained.
Summary of Contents for 4460S
Page 1: ...MLS0002 MANUALE UTENTE USER MANUAL D DE EA A L LU UX X Ref 4460S ITALIANO ENGLISH ...
Page 2: ......
Page 12: ...10 ITALIANO fg 1 CARATTERISTICHE COSTRUTTIVE fg 2 ...
Page 20: ......
Page 37: ......