8
C. Seal the Puncture
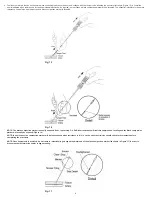
2QFHWKHDQFKRUKDVEHHQGHSOR\HGFRUUHFWO\)LJXUHDQGWKHGHYLFHFDSKDVEHHQORFNHGLQWRWKHUHDUSRVLWLRQ)LJXUHVORZO\DQGFDUHIXOO\ZLWKGUDZWKHGHYLFHVKHDWK
DVVHPEO\DORQJWKHDQJOHRIWKHSXQFWXUHWUDFWWRSRVLWLRQWKHDQFKRUDJDLQVWWKHYHVVHOZDOO)LJXUH
NOTE: Do not re-insert the device. Re-insertion of the device after partial deployment could cause collagen to be deposited in the artery.
Fig. 12
:KHQWKHLQVHUWLRQVKHDWKFOHDUVWKHVNLQDWDPSHUWXEHZLOODSSHDU)LJXUH$WWKLVVWDJHJULSWKHWDPSHUWXEHDQGJHQWO\DGYDQFHWKHNQRWDQGFROODJHQZKLOHPDLQWDLQLQJ
WHQVLRQRQWKHVXWXUH
Fig. 13
WARNING: Failure to maintain tension on the suture while advancing the collagen could cause the collagen to enter the artery.
&RQWLQXHWRZLWKGUDZWKHLQVHUWLRQVKHDWKDQGGHYLFHXQWLOWKHFOHDUVWRSRQWKHVXWXUHDSSHDUV)LJXUH&RQWLQXHWRSXOOXQWLODOORIWKHVXWXUHKDVEHHQGHSOR\HG7KHVXWXUHZLOO
WKHQORFNZLWKLQWKHGHYLFHFDSZKHUHLWLVDWWDFKHG0DLQWDLQWHQVLRQRQWKHVXWXUH
Fig. 14