25
minerals, scale forms. The life giving properties of
water can also encourage biological growth that can
foul heat transfer surfaces.
To avoid the unwanted side effects associated with
water cooling, proper chemical treatment and
preventive maintenance is required for continuous
plant productivity.
Unwanted Side Effects of Improper Water Quality
•
Corrosion
•
Scale
•
Fouling
•
Biological Contamination
Cooling Water Chemistry Properties
•
Electrical Conductivity
•
pH
•
Alkalinity
•
Total Hardness
•
Dissolved gases
Chillers at their simplest have two main heat
exchangers: one that absorbs the heat from the
process (evaporator) and one that removes the heat
from the chiller (condenser). All our chillers use
stainless steel brazed plate evaporators. Our air-
cooled chillers use air to remove heat from the
chiller. These, as are all heat exchangers, are
susceptible to fouling of heat transfer surfaces due
to scale or debris. Fouling of these surfaces reduces
the heat-transfer surface area while increasing the
fluid velocities and pressure drop through the heat
exchanger. All of these effects reduce the heat
transfer and affect the efficiency of the chiller.
The complex nature of water chemistry requires a
specialist to evaluate and implement appropriate
sensing, measurement and treatment needed for
satisfactory performance and life. The
recommendations of the specialist may include
filtration, monitoring, treatment and control devices.
With the ever-changing regulations on water usage
and treatment chemicals, the information is usually
up to date when a specialist in the industry is
involved. Table 1 shows the list of water
characteristics and quality limitations.
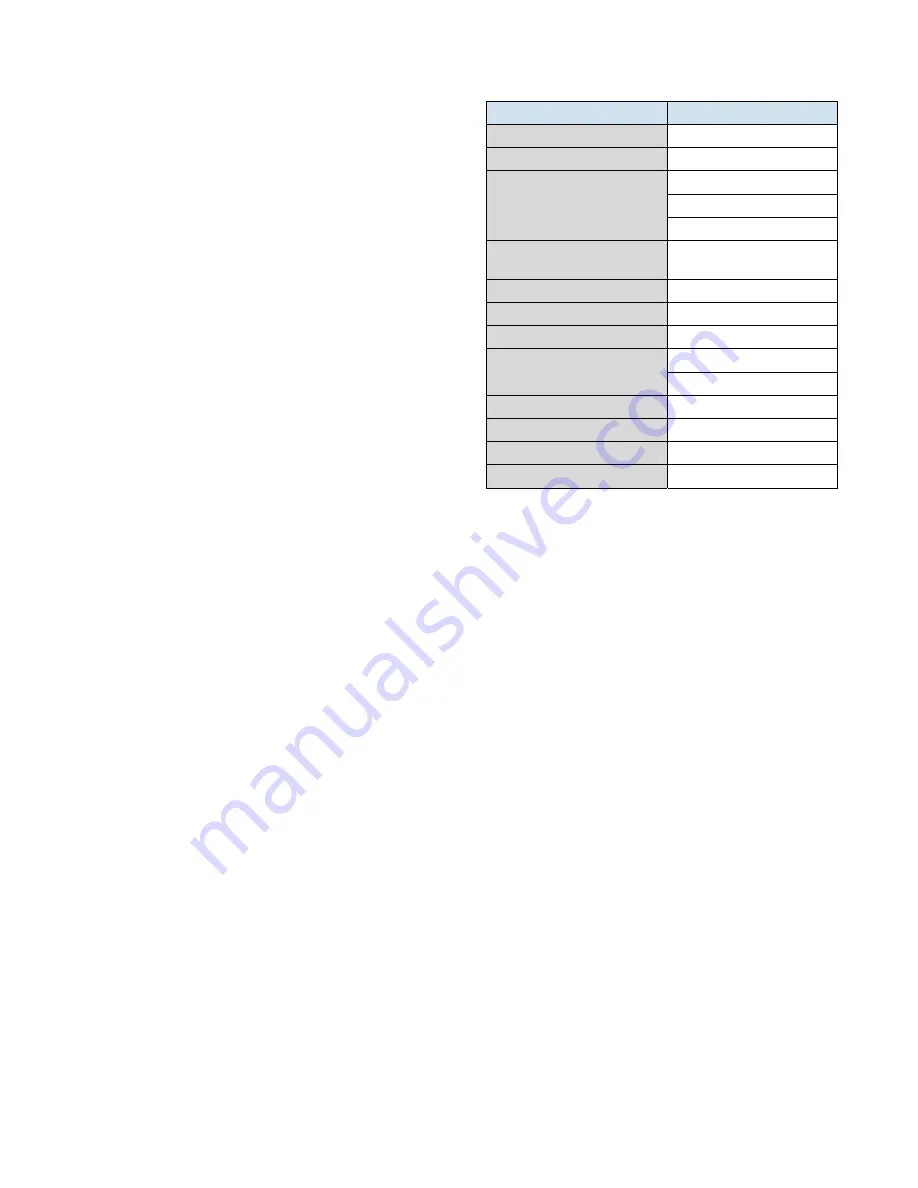
Table 10 – Fill Water Chemistry Requirements
Water Characteristic
Quality Limitation
Alkalinity (HCO
3
-
) 70-300
ppm
Aluminum (Al)
Less than 0.2 ppm
Ammonium (NH
3
)
Less than 2 ppm
Chlorides (Cl
-
)
Less than 300 ppm
Electrical Conductivity
10-500µS/cm
Free (aggressive) Carbon Dioxide
(CO
2
)†
Less than 5 ppm
Free Chlorine(Cl
2
)
Less than 1 PPM
HCO
3
-
/SO
4
2-
Greater than 1.0
Hydrogen Sulfide (H
2
S)*
Less than 0.05 ppm
Iron (Fe)
Less than 0.2 ppm
Manganese (Mn)
Less than 0.1 ppm
Nitrate (NO
3
)
Less than 100 ppm
pH 7.5-9.0
Sulfate (SO
4
2-
)
Less than 70 ppm
Total Hardness (dH)k
4.0-8.5
* Sulfides in the water quickly oxidize when exposed to air;
therefore ensure agitation does not occur when taking a water
sample. Unless tested immediately at the site, the sample will
require stabilization with a few drops of one Molar zinc acetate
solution, allowing accurate sulfide determination up to 24 hours
after sampling. A low pH and high alkalinity cause system
problems, even when both values are within the range shown. The
term pH refers to the acidity, basicity, or neutrality of the water
supply. Below 7.0, water is acidic. Neutral water contains a pH of
7.0.
† Dissolved carbon dioxide calculation is from the pH and total
alkalinity values shown below or measured on the site using a test
kit.
Dissolved Carbon Dioxide, PPM = TA x 2[(6.3-pH)/0.3] where TA =
Total Alkalinity, PPM as CaCO3
Freeze Protection
The chiller includes a flow switch for each fluid circuit
to provide protection of the evaporator from
freezing during a low-flow condition. In addition,
there are safeties in place to protect the evaporator
from freezing due to low fluid temperatures when
the unit is operating. To protect the chiller from
damage caused by freezing in the case of power
failure or a stopped state or in cases when the
anticipated set point temperature is below the
freezing point of water, use an appropriate
concentration of inhibited ethylene or propylene
glycol solution or other suitable inhibited antifreeze
solution. Ensure the antifreeze solution provides
burst protection to a temperature 10°F colder than
the lowest anticipated set point or outside ambient
air temperature.
Summary of Contents for Accuchiller KSE
Page 1: ......
Page 2: ......
Page 10: ...4 Figure 3 Mounting Platform Figure 4 Rigging...
Page 37: ...31 Notes...
Page 38: ...32...
Page 39: ......










































