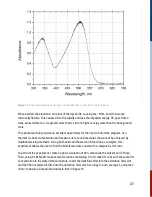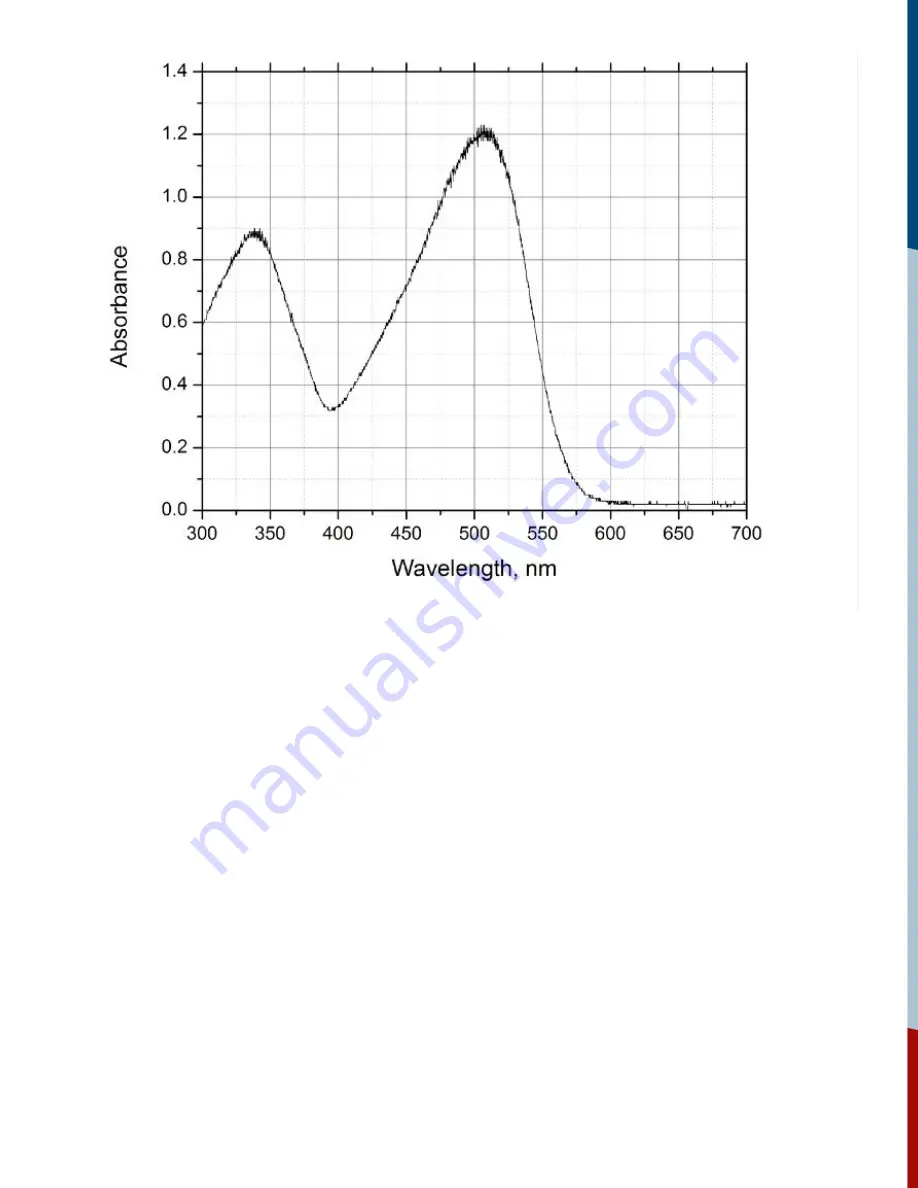
Figure 9:
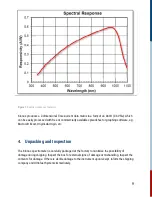
Ground state absorption spectrum of Congo Red (trans conf.) in 20% water/ethanol.
When excited, the electronic structure of the dye shifts, causing the –N=N- bond to become
torsionally flexible. This causes CR to flip rapidly and lose the imparted energy. CR goes from a
trans-excited state to a cis-ground state that is still at a higher energy state than the trans-ground
state.
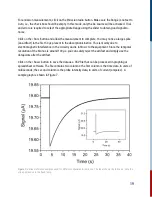
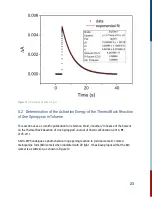
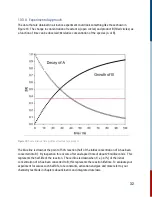
This photo-reaction provides an excellent opportunity for the user to follow the progress of a
thermal cis-trans isomerization and measure its rate on timescales that cannot be achieved by
traditional mixing methods. Using both acids and bases which function as catalysts, this
experiment allows the user to find the bimolecular rate constant for catalysis by OH- ions.
To perform the experiment, create a light red solution of 80% ethanol as the solvent and CR dye.
Then, using 0.1 M NaOH make several solutions containing 0 to 0.1 mM OH- ions with the same CR
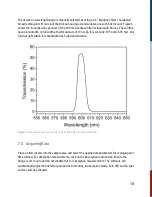
concentration in the water/ethanol mixture. Insert the dark blue filter into the excitation filter slot
and the 580 nm dielectric filter into the detection filter slot. Set range to auto, average 1, and press
<Run> to obtain a time profile similar to that in Figure 10.
21