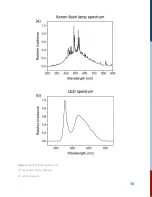
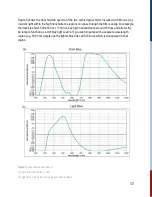
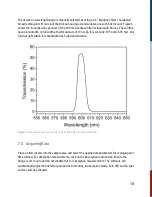
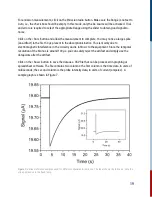
10. Basic Theory
This section aims to provide some background for the user to be aware of flash photolysis
spectroscopy. It does not aim to be a comprehensive guide on the intricacies of the technique and
its physics. For more details and on how to interpret the data, the user is encouraged to read books
on optical spectroscopy and scientific literature.
Most chemical reactions that occur via a series of simple, or elementary processes, which can
proceed at different rates. An elementary process is a single step reaction where there are no
chemically-identifiable intermediates. Elementary processes are best used to understand the
fundamental principles of photochemical kinetics. For a given complex reaction, its rate will be
determined by the rate of the slowest of the participating elementary reactions. The major
advantage of using light to initiate reactions is that in this way it is relatively straightforward to
initiate elementary processes.
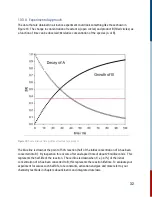
10.1 Transient Optical Spectrometry
Optical spectrometry (sometimes also called optical spectroscopy) is a general term used to
describe measurements done with light, and how matter interacts with light. Depending on the type
of processes that occur, their features and timescales, spectroscopists will choose different
instruments to perform such experiments. The most basic optical spectroscopic techniques are
steady state measurements like absorption or fluorescence spectroscopy. While lacking time
related information, they provide immense spectral information that prompts measurements that
record the change of the sample over time. Such experiments measure the changes of the sample’s
properties, such as absorbance. Measuring the change in absorbance over time is called transient
absorption (TA) spectroscopy.
Transient absorption measurements can range from ‘slow’ to ‘ultrafast’, because different
processes that occur at different speeds, or more accurately, timescales. The faster the processes
the sample undergoes, the shorter the excitation and probe sources need to be, requiring more
expensive instruments. Femtosecond (10 s) TA provides information in the sub-picosecond (10
s) timescales. These timescales are useful to extract information related to electron behavior,
but requires specialized and expensive instrumentation. One of its ‘slow’ counterparts is flash
photolysis, where no specialized equipment is required. To perform such measurements, a
reasonably ‘fast’ pulsed light source and an appropriate detector is sufficient.
TA spectroscopy is also known as pump-probe spectrometry: the pump (in Kronos, the flash lamp)
excites the sample and generates photogenerated carriers that is the interrogated by the probe
−
15
−
12
28