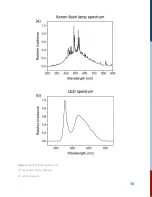
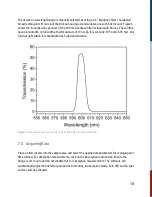
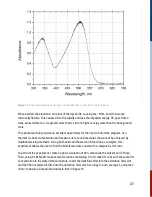
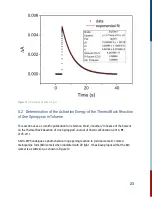
light (LED). The goal is to measure the pump-induced probe change. By taking the difference
between the transmitted and initial signal, and doing a logarithmic calculation, the change in
absorption [ΔA] at a particular wavelength can be obtained,
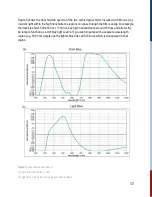
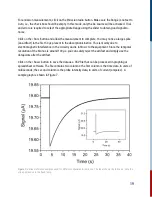
In such measurements, kinetics data is represented in signal vs relative time units, e.g. delay time.
The data is relative to a quantity called ‘time zero’. Time zero describes the instance when the
pump and probe pulses overlap in time at the target, i.e., the sample.
This time zero in flash photolysis is arbitrarily set since it is essentially a single shot measurement.
A distinct feature of time zero is the sudden increase in signal, assuming the pump and probe
beams are also overlapping in space. Depending on the user, it can be just before the signal
amplitude rises, or when the signal is maximum. This manual uses the former definition.
10.2 Photochemistry
Materials in this section have been adapted from “A Qualitative Theory of Molecular Organic
Photochemistry” (
http://www.columbia.edu/itc/chemistry/photochem/courseworks/
). The paper serves as a good introduction to photochemistry. Interested and
curious readers are strongly encouraged to read it in its entirety.
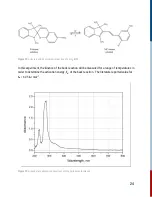
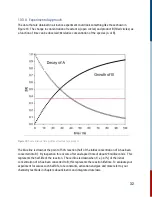
When a molecule
R
, the reactant, absorbs energy (
hv
), it undergoes a photophysical process to an
excited molecule (
*R
). The excited molecule can then relax back to the ground state non-radiatively
(photophysical). It can also undergo photochemical processes to form a product (
P
) in a single
step or via an intermediate (
I
). One can think of photophysical processes that do not change the
sample and reversible, whereas photochemical processes result in a different chemical outcome
and are generally irreversible.
Figure 17:
Photophysical and photochemical sequence in light-matter interactions.
29