80
03-914875-00:1
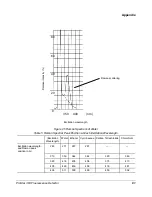
Raman Scattering
When fluorescence is measured, two additional peaks may
appear in the spectrum. The Rayleigh peak appears at the
excitation wavelength and is due to scattered light, while the
Raman peak appears at longer wavelength than the excitation.
The position of the Raman band is dependent on the excitation
wavelength, while the position of the fluorescence is independent
of the excitation wavelength.
The Rayleigh peak and the Raman peak will occur even if the
eluent does not contain any compound that fluoresces. If there
is any doubt whether an observed peak is a Raman peak, a
Rayleigh peak or a peak due to the fluorescence from the
compound of interest, simply change the excitation wavelength
slightly.
If the peak is due to the Raman effect or the Rayleigh effect, the
observed wavelength will shift. If the peak is due to fluorescence
from the compound of interest the wavelength will not change
(although the peak height may change).
The Raman effect is moderately strong when water is the
solvent, but is considerably weaker for other solvents that are
commonly used in HPLC.
Table 5 presents the position of the Raman peak for a variety of
excitation wavelengths.
Summary of Contents for ProStar 363
Page 2: ......
Page 6: ...iv 03 914875 00 1...
Page 20: ...14 03 914875 00 1...
Page 82: ...76 03 914875 00 1...