Villa Sistemi Medicali
ARCOVIS 3000 S/R
User's Manual
[File:201175-G-01-01.doc]
Rev. E - Pag. 7/76
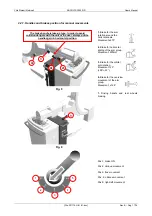
1.10. Classification
Protection against electrical hazards ............. Class I
Protection against direct and indirect contact Unit Type B with Type B applied part
Protection against water penetration.............. Common protection (IPXO)
Fluoroscopy footswitch protected against the
submersion effects (IPX8)
Use condition protection................................. Continuous working with temporary load
1.11. List of the Standards for the evaluation of the product compliance
Reference
Description
MDD 93/42/EEC class IIB according to
Annex IX rule 10.
Medical Devices Directive (EC mark)
IEC 60601-1 1st edition
Medical devices safety
IEC 60601-1-2 1st edition
Electromagnetic compatibility
IEC 60601-1-3 1st edition
Protection against ionizing radiation
IEC 60601-2-7 2nd edition
HV generators
IEC 60601-2-28 1st edition
Tube-housing groups
IEC 60601-2-32 1st edition
Mechanical safety aspects
ISO 14971:2000
Risk analysis
CEI EN 60825-1 2nd edition
Laser equipment safety
ARCOVIS 3000 S/R with radio-protection according to the Standard CEI EN 60601-1-3 (1995)
Gruppo Inverter-monoblocco:
(IN-9040-5 HF + I-40S 3,5 RF, IN-9040-5 HF + I-40R 5 RF) EN60601-2-7:1998
X-ray group for diagnostics ARCOVIS 3000 S/R IEC 601-2-28 (1993)
Complementary unit ARCOVIS 3000 S/R IEC 601-2-32
1.12. Compliance
This x-ray unit is in compliance with the Electromedical Devices Directive
93/42 EEC class
IIb
and with the Annex IX rule 10.
For any further information concerning the
compliance please contact:
The manufacturer (according to the European
Directive 93/42/EEC) of the unit ARCOVIS
3000 S/R is:
Villa Sistemi Medicali
Via delle Azalee, 3
20090 Buccinasco, MI (ITALY)
Tel: +39-02-48.859.1
Fax: +39-02-48.81.844
E-mail:
Technix S.p.A.
Via E. Fermi, 45
24050 Grassobbio, BG (ITALY)
Tel: +39 (0)35-3846611
Fax: +39 (0)35-335675