Electrochemical Corrosion
General
NOTICE!
Refer to the Service handbook
Corrosion
measurement, DPH/DPR & IPS
for further information.
Corrosion theory
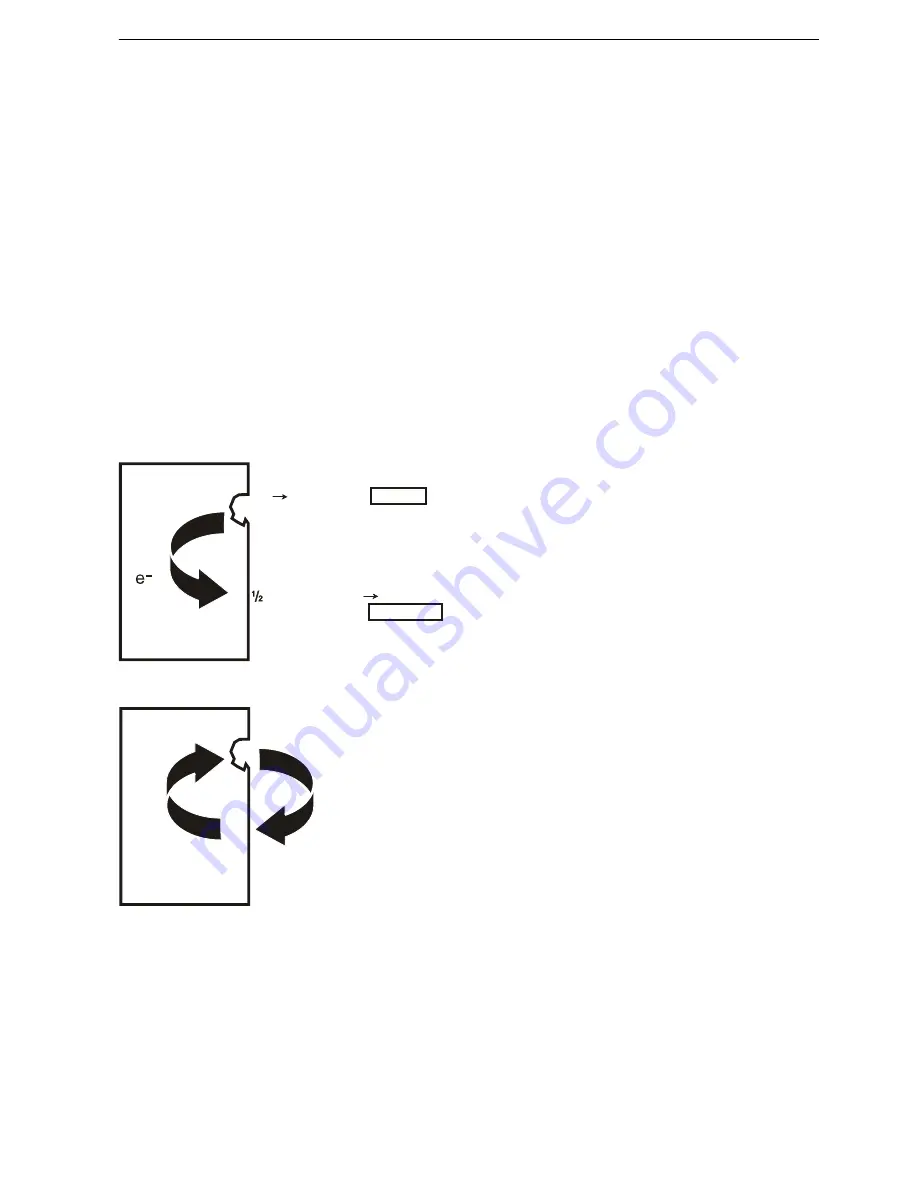
Corrosion in water is always electrochemical in nature.
This means that a weak electric current occurs at the
same time as chemical reactions takes place. Two
chemical reactions are required to make a metal cor-
rode, an oxidation reaction (metal dissolving) and a
reduction reaction (generally oxygen consuming). Oxi-
dation is referred to as an anode reaction and reduc-
tion is referred to as a cathodic reaction. In an oxidation
reaction, electrons are freed which are transported in
the metal to another point, where they are consumed
in a cathodic reaction.
Electrons are thus transported in the metal from the
anode to the cathode. This causes a weak DC current
in the opposite direction. An electric circuit must be
closed. This is achieved by the transport of ions in the
water.
Anodic and cathodic reactions must always balance
each other, which means that the electrons released
at the anode must be consumed at the cathode. If the
anodic and cathodic reactions occur evenly distributed
across the entire surface, general corrosion occurs.
The depth of attack then becomes basically equal
across the entire surface. This commonly occurs on
steel and bronze.
Fe Fe2+ +2 e-
O2 + H2O + 2 e 2 OH-
ANODE
CATHODE
P0011416
I
P0011417
Arrangement and Planning, Electrochemical Corrosion
47704162 10-2014 © AB VOLVO PENTA
33
Summary of Contents for IPS650
Page 1: ...IPS 2 IPS650 IPS800 IPS950 Installation 1 1 E ...
Page 2: ......
Page 156: ......
Page 160: ...47704162 English 10 2014 ...













































