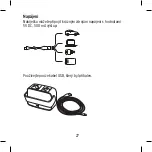
SYMBOLS
Symbols commonly used in medical device labelling (labels/IFU/etc.)
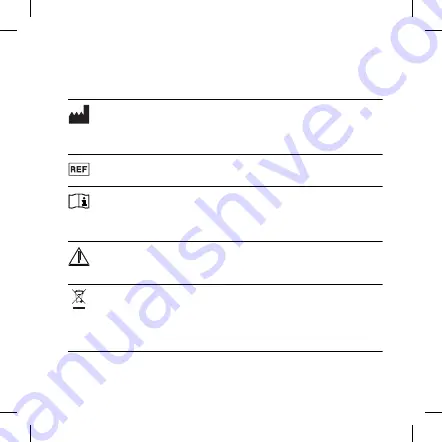
Symbol
Title/Description
Manufacturer
The product is produced by the manufacturer whose name and ad-
dress are stated next to the symbol. If appropriate, the date of manu-
facture may also be stated.
Catalogue number
The product’s catalogue (item) number.
Consult instructions for use
The user instructions contain important cautionary information
(warnings/precautions) and must be read before using the prod-
uct.
Warning
Text marked with a warning symbol must be read before using
the product.
WEEE mark
“Not for general waste”. When a product is to be discarded, it
must be sent to a designated collection point for recycling and re-
covering to prevent the risk of harm to the environment or hu-
man health as a result of the presence of hazardous substances.
21