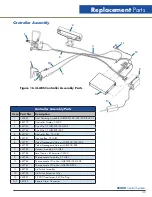
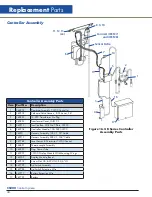
CS200
Control Systems
54
Appendix
EC Declaration of Conformity
The undersigned representing the manufacturer:
Pacer Digital Systems, Inc.
Attn: Kevin Oeff
8658 Castle Park Drive, Suite 103
Indianapolis, IN 46256, USA
Herewith declared that the Product:
LN
2
Level Control for Cryostorage System
Model/Type ref.:
CS CONTROL SYSTEM
is in conformity with the Essential requirements of the following EC Directives when subject
to correct installation, maintenance and use conforming to its(their)intended purpose, to the
applicable regulations and standards, to our operation and maintenance manual.
93/42/EEC Medical Device Directive
2004/108/EC EMC Directive
2006/95/EC Low Voltage Directive
and that the Standards and/or technical specifications referenced below
have been applied:
• EN 60601-1:2006+A11:2001 Medical Electrical Equipment – General Requirements
for basic safety and essential performance.
• IEC 61010-1:2001 (Second Edition)- Safety requirements for electrical equipment for
measurement, control, and laboratory use Part 1: General requirements
• IEC 60601-1-2: 2007 Edition 3 Medical Electrical Equipment – General Requirements for basic safety and
essential performance – Collateral standard : Electromagnetic Compatibility
• IEC/CISPR 11:2009+A1:2010 Radiated & Conducted Emissions.
• IEC61000-3-2:2005+A1:2008+A2:2009. Harmonics
• IEC 61000-3-3:2008. Flicker
Year of CE Marking: 2012
Manufacturer:
Pacer Digital Systems, Inc.
Signature:
Kevin Oeff
(Digitally signed by Kevin Oeff)
Position:
President
Date:
24 May 2012
Place:
Indianapolis, IN USA
Summary of Contents for CS200
Page 2: ......